Effects of ICI 182780 on estrogen receptor expression, fluid absorption and sperm motility in the epididymis of the bonnet monkey
- PMID: 15743524
- PMCID: PMC1079944
- DOI: 10.1186/1477-7827-3-10
Effects of ICI 182780 on estrogen receptor expression, fluid absorption and sperm motility in the epididymis of the bonnet monkey
Abstract
Background: The importance of estrogen in regulation of fluid absorption and sperm maturation in the rodent epididymis has been established from studies on estrogen receptor-alpha knockout mice. However, functional studies on the role of estrogen in primate epididymis have been few. The main objective of this study was therefore to extend these observations and systematically analyze the presence and function of estrogen receptors in modulating the function of the primate epididymis, using the bonnet monkey (Macaca radiata) as a model system.
Methods: A steroidal estrogen receptor (ER) antagonist, ICI 182780 (ICI), was administered to adult male bonnet monkeys via mini-osmotic pumps for a duration of 30 to 180 days. The expression of key estrogen-regulated genes (ER-alpha, Na-K ATPase alpha-1 and Aquaporin-1) was examined at specific time points. Further, the effect of ICI in modulating fluid reabsorption in efferent ductules was monitored, and critical sperm-maturation parameters were also analyzed.
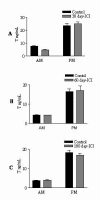
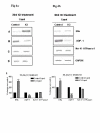
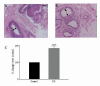
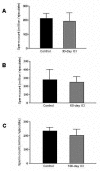
Results: Our studies in the bonnet monkey revealed that both ER-alpha and ER-beta were expressed in all the three regions of the epididymis. We observed an increase in ER-alpha mRNA and protein in the caput of ICI-treated monkeys. Steady state mRNA levels of the water-channel protein, Aquaporin-1, was significantly lower in the caput of ICI-treated monkeys compared to controls, whereas the mRNA levels of Na-K ATPase alpha-1 remained unchanged. In vitro incubation of efferent ductules with ICI resulted in two-fold increase in tubular diameter, indicating affected fluid reabsorption capacity. Furthermore, sperm from ICI-treated monkeys were immotile.
Conclusion: Taken together, our results point to an integral role for estrogen in modulating the functions of the bonnet monkey epididymis. This study also demonstrates possible differences in the epididymal physiology of rodents and non-human primates, and thus underscores the significance of reports such as these, that examine the physiology of non-human primates (as opposed to rodents), in an attempt to understand similar events in the human.
Figures



Similar articles
-
Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ER beta.Biol Reprod. 2001 Nov;65(5):1534-41. doi: 10.1095/biolreprod65.5.1534. Biol Reprod. 2001. PMID: 11673272
-
The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy.Reprod Biol Endocrinol. 2003 Aug 18;1:57. doi: 10.1186/1477-7827-1-57. Reprod Biol Endocrinol. 2003. PMID: 12959643 Free PMC article.
-
Expression of aquaporins in the efferent ductules, sperm counts, and sperm motility in estrogen receptor-alpha deficient mice fed lab chow versus casein.Mol Reprod Dev. 2006 Feb;73(2):226-37. doi: 10.1002/mrd.20390. Mol Reprod Dev. 2006. PMID: 16261609 Free PMC article.
-
Delineating the role of estrogen in regulating epididymal gene expression.Soc Reprod Fertil Suppl. 2007;63:31-43. Soc Reprod Fertil Suppl. 2007. PMID: 17566259 Review.
-
Estrogen and its receptors in efferent ductules and epididymis.J Androl. 2011 Nov-Dec;32(6):600-13. doi: 10.2164/jandrol.110.012872. Epub 2011 Mar 25. J Androl. 2011. PMID: 21441425 Review.
Cited by
-
Estrogen, efferent ductules, and the epididymis.Biol Reprod. 2011 Feb;84(2):207-17. doi: 10.1095/biolreprod.110.087353. Epub 2010 Oct 6. Biol Reprod. 2011. PMID: 20926801 Free PMC article. Review.
-
Urinary bisphenol A levels in young men: association with reproductive hormones and semen quality.Environ Health Perspect. 2014 May;122(5):478-84. doi: 10.1289/ehp.1307309. Epub 2014 May 1. Environ Health Perspect. 2014. PMID: 24786630 Free PMC article.
-
Expressional Modulation of Aquaporin 1 and 9 in the Rat Epididymis by an Anabolic-Androgenic Steroid, Nandrolone Decanoate.Dev Reprod. 2021 Dec;25(4):245-255. doi: 10.12717/DR.2021.25.4.245. Epub 2021 Dec 31. Dev Reprod. 2021. PMID: 35141450 Free PMC article.
-
Disruption of estrogen receptor signaling and similar pathways in the efferent ductules and initial segment of the epididymis.Spermatogenesis. 2014 Dec 31;4(2):e979103. doi: 10.4161/21565562.2014.979103. eCollection 2014 May-Aug. Spermatogenesis. 2014. PMID: 26413389 Free PMC article. Review.
-
Tissue physiology and pathology of aromatase.Steroids. 2012 Jan;77(1-2):27-35. doi: 10.1016/j.steroids.2011.10.013. Epub 2011 Nov 13. Steroids. 2012. PMID: 22108547 Free PMC article. Review.
References
-
- Turner TT. On the epididymis and its role in the development of the fertile ejaculate. J Androl. 1995;16:292–298. - PubMed
-
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor α has a functional role in the mouse rete testis and efferent ductules. Biol Reprod. 2000;63:1873–1880. - PubMed
-
- Hess RA, Zhou Q, Nie R. The role of estrogens in the endocrine and paracrine regulation of the efferent ductules, epididymis and vas deferens. In: Bernard R, Hinton B, editor. The Epididymis from molecules to clinical practice. New York: Kluwer Academic/Plenum publishers; 2002. pp. 317–337.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

