Pharmacokinetics of isoflavones, daidzein and genistein, after ingestion of soy beverage compared with soy extract capsules in postmenopausal Thai women
- PMID: 15743537
- PMCID: PMC555741
- DOI: 10.1186/1472-6904-5-2
Pharmacokinetics of isoflavones, daidzein and genistein, after ingestion of soy beverage compared with soy extract capsules in postmenopausal Thai women
Abstract
Background: Isoflavones from soybeans may provide some beneficial impacts on postmenopausal health. The purpose of this study was to compare the pharmacokinetics and bioavailability of plasma isoflavones (daidzein and genistein) after a single dose of orally administered soy beverage and soy extract capsules in postmenopausal Thai women.
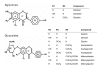
Methods: We conducted a randomized two-phase crossover pharmacokinetic study in 12 postmenopausal Thai women. In the first phase, each subject randomly received either 2 soy extract capsules (containing daidzin : genistin = 7.79 : 22.57 mg), or soy beverage prepared from 15 g of soy flour (containing daidzin : genistin = 9.27 : 10.51 mg). In the second phase, the subjects received an alternative preparation in the same manner after a washout period of at least 1 week. Blood samples were collected immediately before and at 0.5, 1, 2, 4, 6, 8, 10, 12, 24 and 32 h after administration of the soy preparation in each phase. Plasma daidzein and genistein concentrations were determined by using high performance liquid chromatography (HPLC). The pharmacokinetic parameters of daidzein and genistein, i.e. maximal plasma concentration (Cmax), time to maximal plasma concentration (Tmax), area under the plasma concentration-time curve (AUC) and half-life (t1/2), were estimated using the TopFit version 2.0 software with noncompartmental model analysis.
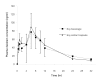
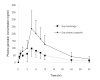
Results: There were no significant differences in the mean values of Cmax/dose, AUC0-32/dose, AUC0- proportional, variant/dose, Tmax, and t1/2 of genistein between both preparations. For pharmacokinetic parameters of daidzein, the mean values of Cmax/dose, Tmax, and t1/2 did not significantly differ between both preparations. Nonetheless, the mean AUC0-32/dose and AUC0- proportional, variant/dose after administration of soy extract capsules were slightly (but significantly, p < 0.05) higher than those of soy beverage.
Conclusion: The bioavailability of daidzein, which was adjusted for the administered dose (AUC/dose), following a single oral administration of soy beverage was slightly (but significantly) less than that of soy extract capsules, whereas, the bioavailability adjusted for administered dose of genistein from both soy preparations were comparable. The other pharmacokinetic parameters of daidzein and genistein, including Cmax adjusted for the dose, Tmax and t1/2, were not different between both soy preparations.
Figures
References
-
- Schneider HPG. Menopause: The state of the art in research and management. New York: The Parthenon Publishing; 2003.
-
- Song T, Barua K, Buseman G, Murphy PA. Soy isoflavone analysis: quality control and a new internal standard. Am J Clin Nutr. 1998;68:1474–1479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

