Relationship of acute lung inflammatory injury to Fas/FasL system
- PMID: 15743781
- PMCID: PMC1602343
- DOI: 10.1016/S0002-9440(10)62290-0
Relationship of acute lung inflammatory injury to Fas/FasL system
Abstract
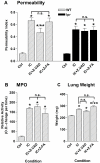
There is mounting evidence that apoptosis plays a significant role in tissue damage during acute lung injury. To evaluate the role of the apoptosis mediators Fas and FasL in acute lung injury, Fas (lpr)- or FasL (gld)-deficient and wild-type mice were challenged with intrapulmonary deposition of IgG immune complexes. Lung injury parameters ((125)I-albumin leak, accumulation of myeloperoxidase, and wet lung weights) were measured and found to be consistently reduced in both lpr and gld mice. In wild-type mice, lung injury was associated with a marked increase in Fas protein in lung. Inflamed lungs of wild-type mice showed striking evidence of activated caspase-3, which was much diminished in inflamed lungs from lpr mice. Intratracheal administration of a monoclonal Fas-activating antibody (Jo2) in wild-type mice induced MIP-2 and KC production in bronchoalveolar lavage fluids, and a murine alveolar macrophage cell line (MH-S) showed significantly increased MIP-2 production after incubation with this antibody. Bronchoalveolar lavage fluid content of MIP-2 and KC was substantially reduced in lpr mice after lung injury when compared to levels in wild-type mice. These data suggest that the Fas/FasL system regulates the acute lung inflammatory response by positively affecting CXC-chemokine production, ultimately leading to enhanced neutrophil influx and tissue damage.
Figures







Similar articles
-
Role of the Fas/FasL system in a model of RSV infection in mechanically ventilated mice.Am J Physiol Lung Cell Mol Physiol. 2011 Oct;301(4):L451-60. doi: 10.1152/ajplung.00368.2010. Epub 2011 Jul 8. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21743025 Free PMC article.
-
Divergent Effects of Neutrophils on Fas-Induced Pulmonary Inflammation, Apoptosis, and Lung Damage.Shock. 2017 Feb;47(2):225-235. doi: 10.1097/SHK.0000000000000685. Shock. 2017. PMID: 27405068
-
Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors.J Immunol. 2001 Dec 1;167(11):6217-24. doi: 10.4049/jimmunol.167.11.6217. J Immunol. 2001. PMID: 11714783
-
Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria.Infect Immun. 2001 Sep;69(9):5768-76. doi: 10.1128/IAI.69.9.5768-5776.2001. Infect Immun. 2001. PMID: 11500454 Free PMC article.
-
Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation.Am J Pathol. 2001 Jan;158(1):153-61. doi: 10.1016/S0002-9440(10)63953-3. Am J Pathol. 2001. PMID: 11141488 Free PMC article.
Cited by
-
The Pla protease of Yersinia pestis degrades fas ligand to manipulate host cell death and inflammation.Cell Host Microbe. 2014 Apr 9;15(4):424-34. doi: 10.1016/j.chom.2014.03.005. Cell Host Microbe. 2014. PMID: 24721571 Free PMC article.
-
Sodium hydrosulfide alleviates lung inflammation and cell apoptosis following resuscitated hemorrhagic shock in rats.Acta Pharmacol Sin. 2013 Dec;34(12):1515-25. doi: 10.1038/aps.2013.96. Epub 2013 Oct 14. Acta Pharmacol Sin. 2013. PMID: 24122010 Free PMC article.
-
Lung Expression of Macrophage Markers CD68 and CD163, Angiotensin Converting Enzyme 2 (ACE2), and Caspase-3 in COVID-19.Medicina (Kaunas). 2023 Apr 6;59(4):714. doi: 10.3390/medicina59040714. Medicina (Kaunas). 2023. PMID: 37109672 Free PMC article.
-
Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis.Am J Physiol Renal Physiol. 2009 Jul;297(1):F125-37. doi: 10.1152/ajprenal.90666.2008. Epub 2009 Apr 29. Am J Physiol Renal Physiol. 2009. PMID: 19403643 Free PMC article.
-
The role of complement in danger sensing and transmission.Immunol Res. 2006;34(2):157-76. doi: 10.1385/IR:34:2:157. Immunol Res. 2006. PMID: 16760575 Review.
References
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. - PubMed
-
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. - PubMed
-
- Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. - PubMed
-
- Artigas A, Carlet J, Le Gall JR, Chastang CL, Blanch L, Fernandez R. Clinical presentation, prognostic factors and outcome of ARDS in the European Collaborative Study (1985–1987). Zapol WM, Lemaire F, editors. New York: Marcel Dekker, Inc.,; Adult Respiratory Distress Syndrome. 1991:pp 37–63.
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

