A cyclosporine-sensitive psoriasis-like disease produced in Tie2 transgenic mice
- PMID: 15743796
- PMCID: PMC1602355
- DOI: 10.1016/S0002-9440(10)62305-X
A cyclosporine-sensitive psoriasis-like disease produced in Tie2 transgenic mice
Abstract
Psoriasis is a common, persistent skin disorder characterized by recurrent erythematous lesions thought to arise as a result of inflammatory cell infiltration and activation of keratinocyte proliferation. Unscheduled angiogenic growth has also been proposed to mediate the pathogenesis of psoriasis although the cellular and molecular basis for this response remains unclear. Recently, a role for the angiopoietin signaling system in psoriasis has been suggested by studies that demonstrate an up-regulation of the tyrosine kinase receptor Tie2 (also known as Tek) as well as angiopoietin-1 and angiopoietin-2 in human psoriatic lesions. To examine temporal expression of Tie2, we have developed a binary transgenic approach whereby expression of Tie2 can be conditionally regulated by the presence of tetracycline analogs in double-transgenic mice. A psoriasis-like phenotype developed in double-transgenic animals within 5 days of birth and persisted throughout adulthood. The skin of affected mice exhibited many cardinal features of human psoriasis including epidermal hyperplasia, inflammatory cell accumulation, and altered dermal angiogenesis. These skin abnormalities resolved completely with tetracycline-mediated suppression of transgene expression, thereby illustrating a complete dependence on Tie2 signaling for disease maintenance and progression. Furthermore, the skin lesions in double-transgenic mice markedly improved after administration of the immunosuppressive anti-psoriatic agent cyclosporine, thus demonstrating the clinical significance of this new model.
Figures

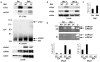
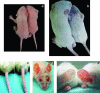


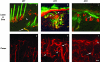


Similar articles
-
Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis.Am J Pathol. 2009 Apr;174(4):1443-58. doi: 10.2353/ajpath.2009.080858. Am J Pathol. 2009. PMID: 19342373 Free PMC article.
-
Resolution of endothelial activation and down-regulation of Tie2 receptor in psoriatic skin after infliximab therapy.J Am Acad Dermatol. 2006 Jun;54(6):1003-12. doi: 10.1016/j.jaad.2006.01.038. Epub 2006 Mar 20. J Am Acad Dermatol. 2006. PMID: 16713454
-
Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis.J Invest Dermatol. 2001 May;116(5):713-20. doi: 10.1046/j.1523-1747.2001.01316.x. J Invest Dermatol. 2001. PMID: 11348459
-
Angiopoietins/Tie2 signaling axis and its role in angiogenesis of psoriasis.Acta Histochem. 2025 Mar;127(1):152228. doi: 10.1016/j.acthis.2024.152228. Epub 2025 Jan 2. Acta Histochem. 2025. PMID: 39752990 Review.
-
The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development.Semin Cell Dev Biol. 2002 Feb;13(1):19-27. doi: 10.1006/scdb.2001.0288. Semin Cell Dev Biol. 2002. PMID: 11969368 Review.
Cited by
-
Characterization of a Porcine Model for Von Willebrand Disease Type 1 and 3 Regarding Expression of Angiogenic Mediators in the Nonpregnant Female Reproductive Tract.Comp Med. 2019 Oct 1;69(5):401-412. doi: 10.30802/AALAS-CM-19-000003. Epub 2019 Sep 16. Comp Med. 2019. PMID: 31526432 Free PMC article.
-
Immunopathogenesis of psoriasis.Clin Rev Allergy Immunol. 2007 Oct;33(1-2):45-56. doi: 10.1007/s12016-007-0039-2. Clin Rev Allergy Immunol. 2007. PMID: 18094946 Review.
-
Nephroprotective Potential of Mesenchymal Stromal Cells and Their Extracellular Vesicles in a Murine Model of Chronic Cyclosporine Nephrotoxicity.Front Cell Dev Biol. 2020 May 5;8:296. doi: 10.3389/fcell.2020.00296. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32432111 Free PMC article.
-
Psoriasis: what we have learned from mouse models.Nat Rev Rheumatol. 2010 Dec;6(12):704-14. doi: 10.1038/nrrheum.2010.157. Epub 2010 Sep 28. Nat Rev Rheumatol. 2010. PMID: 20877306 Review.
-
Angiogenesis in psoriasis and psoriatic arthritis: clues to disease pathogenesis.Curr Rheumatol Rep. 2005 Aug;7(4):325-9. doi: 10.1007/s11926-005-0044-5. Curr Rheumatol Rep. 2005. PMID: 16045837 Review.
References
-
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. - PubMed
-
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. - PubMed
-
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. - PubMed
-
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. - PubMed
-
- Ward N, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin Cell Dev Biol. 2002;1:19–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

