Transcription factor interactions and chromatin modifications associated with p53-mediated, developmental repression of the alpha-fetoprotein gene
- PMID: 15743813
- PMCID: PMC1061614
- DOI: 10.1128/MCB.25.6.2147-2157.2005
Transcription factor interactions and chromatin modifications associated with p53-mediated, developmental repression of the alpha-fetoprotein gene
Abstract
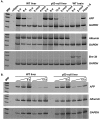
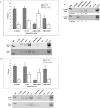
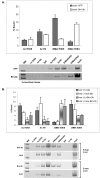
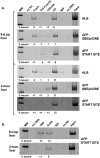
We performed chromatin immunoprecipitation (ChIP) analyses of developmentally staged solid tissues isolated from wild-type and p53-null mice to determine specific histone N-terminal modifications, histone-modifying proteins, and transcription factor interactions at the developmental repressor region (-850) and core promoter of the hepatic tumor marker alpha-fetoprotein (AFP) gene. Both repression of AFP during liver development and silencing in the brain, where AFP is never expressed, are associated with dimethylation of histone H3 lysine 9 (DiMetH3K9) and the presence of heterochromatin protein 1 (HP1). These heterochromatic markers remain localized to AFP during developmental repression but spread to the upstream albumin gene during silencing. Developmentally regulated decreases in levels of acetylated H3 (AcH3K9) and H4 (AcH4) and of di- and trimethylated H3K4 (DiMetH3K4 and TriMetH3K4) occur at both the core promoter and distal repressor regions of AFP. Hepatic expression of AFP correlates with FoxA interaction at the repressor region and the binding of RNA polymerase II and TATA-binding protein to the core promoter. p53 acts as a developmental repressor of AFP in the liver by binding to chromatin, excluding FoxA interaction and targeting mSin3A/HDAC1 to the distal repressor region. p53-null mice exhibit developmentally delayed AFP repression, concomitant with acetylation of H3K9, methylation of H3K4, and loss of DiMetH3K9, mSin3A/HDAC1, and HP1 interactions.
Figures


Similar articles
-
A direct intersection between p53 and transforming growth factor beta pathways targets chromatin modification and transcription repression of the alpha-fetoprotein gene.Mol Cell Biol. 2005 Feb;25(3):1200-12. doi: 10.1128/MCB.25.3.1200-1212.2005. Mol Cell Biol. 2005. PMID: 15657445 Free PMC article.
-
Family members p53 and p73 act together in chromatin modification and direct repression of alpha-fetoprotein transcription.J Biol Chem. 2005 Nov 25;280(47):39152-60. doi: 10.1074/jbc.M504655200. Epub 2005 Oct 2. J Biol Chem. 2005. PMID: 16203738
-
p53 targets chromatin structure alteration to repress alpha-fetoprotein gene expression.J Biol Chem. 2001 Nov 9;276(45):42057-62. doi: 10.1074/jbc.C100381200. Epub 2001 Sep 25. J Biol Chem. 2001. PMID: 11572852
-
Maintaining memory of silencing at imprinted differentially methylated regions.Cell Mol Life Sci. 2016 May;73(9):1871-9. doi: 10.1007/s00018-016-2157-6. Epub 2016 Feb 16. Cell Mol Life Sci. 2016. PMID: 26883803 Free PMC article. Review.
-
Histone deacetylases: silencers for hire.Trends Biochem Sci. 2000 Mar;25(3):121-6. doi: 10.1016/s0968-0004(00)01551-6. Trends Biochem Sci. 2000. PMID: 10694882 Review.
Cited by
-
Role of Ucp1 enhancer methylation and chromatin remodelling in the control of Ucp1 expression in murine adipose tissue.Diabetologia. 2010 Jun;53(6):1164-73. doi: 10.1007/s00125-010-1701-4. Epub 2010 Mar 18. Diabetologia. 2010. PMID: 20238096 Free PMC article.
-
Global analysis of in vivo Foxa2-binding sites in mouse adult liver using massively parallel sequencing.Nucleic Acids Res. 2008 Aug;36(14):4549-64. doi: 10.1093/nar/gkn382. Epub 2008 Jul 8. Nucleic Acids Res. 2008. PMID: 18611952 Free PMC article.
-
Tumor suppressor protein p53 recruits human Sin3B/HDAC1 complex for down-regulation of its target promoters in response to genotoxic stress.PLoS One. 2011;6(10):e26156. doi: 10.1371/journal.pone.0026156. Epub 2011 Oct 20. PLoS One. 2011. PMID: 22028823 Free PMC article.
-
Genome-wide location analysis reveals distinct transcriptional circuitry by paralogous regulators Foxa1 and Foxa2.PLoS Genet. 2012;8(6):e1002770. doi: 10.1371/journal.pgen.1002770. Epub 2012 Jun 21. PLoS Genet. 2012. PMID: 22737085 Free PMC article.
-
Postnatal liver functional maturation requires Cnot complex-mediated decay of mRNAs encoding cell cycle and immature liver genes.Development. 2019 Feb 15;146(4):dev168146. doi: 10.1242/dev.168146. Development. 2019. PMID: 30733279 Free PMC article.
References
-
- Agalioti, T., S. Lomvardas, B. Parekh, J. Yie, T. Maniatis, and D. Thanos. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell 103:667-678. - PubMed
-
- Camper, S. A., R. Godbout, and S. M. Tilghman. 1989. The developmental regulation of albumin and alpha-fetoprotein gene expression. Prog. Nucleic Acid Res. Mol. Biol. 36:131-143. - PubMed
-
- Camper, S. A., and S. M. Tilghman. 1991. The activation and silencing of gene transcription in the liver. Bio/Technology 16:81-87. - PubMed
-
- Camper, S. A., and S. M. Tilghman. 1989. Postnatal repression of the alpha-fetoprotein gene is enhancer independent. Genes Dev. 3:537-546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
