Removal of C-terminal SRC kinase from the immune synapse by a new binding protein
- PMID: 15743820
- PMCID: PMC1061611
- DOI: 10.1128/MCB.25.6.2227-2241.2005
Removal of C-terminal SRC kinase from the immune synapse by a new binding protein
Abstract
The Csk tyrosine kinase negatively regulates the Src family kinases Lck and Fyn in T cells. Engagement of the T-cell antigen receptor results in a removal of Csk from the lipid raft-associated transmembrane protein PAG/Cbp. Instead, Csk becomes associated with an approximately 72-kDa tyrosine-phosphorylated protein, which we identify here as G3BP, a phosphoprotein reported to bind the SH3 domain of Ras GTPase-activating protein. G3BP reduced the ability of Csk to phosphorylate Lck at Y505 by decreasing the amount of Csk in lipid rafts. As a consequence, G3BP augmented T-cell activation as measured by interleukin-2 gene activation. Conversely, elimination of endogenous G3BP by RNA interference increased Lck Y505 phosphorylation and reduced TCR signaling. In antigen-specific T cells, endogenous G3BP moved into a intracellular location adjacent to the immune synapse, but deeper inside the cell, upon antigen recognition. Csk colocalization with G3BP occurred in this "parasynaptic" location. We conclude that G3BP is a new player in T-cell-antigen receptor signaling and acts to reduce the amount of Csk in the immune synapse.
Figures
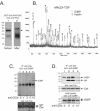


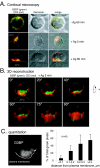
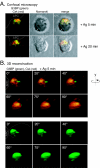
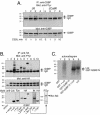

Similar articles
-
Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells.J Immunol. 2002 Sep 15;169(6):2813-7. doi: 10.4049/jimmunol.169.6.2813. J Immunol. 2002. PMID: 12218089
-
Knockdown of C-terminal Src kinase by siRNA-mediated RNA interference augments T cell receptor signaling in mature T cells.Eur J Immunol. 2004 Aug;34(8):2191-9. doi: 10.1002/eji.200425036. Eur J Immunol. 2004. PMID: 15259016
-
Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor.Mol Cell Biol. 2003 Mar;23(6):2017-28. doi: 10.1128/MCB.23.6.2017-2028.2003. Mol Cell Biol. 2003. PMID: 12612075 Free PMC article.
-
Spatiotemporal control of cyclic AMP immunomodulation through the PKA-Csk inhibitory pathway is achieved by anchoring to an Ezrin-EBP50-PAG scaffold in effector T cells.FEBS Lett. 2010 Jun 18;584(12):2681-8. doi: 10.1016/j.febslet.2010.04.056. Epub 2010 Apr 24. FEBS Lett. 2010. PMID: 20420835 Review.
-
Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation.Oncogene. 2004 Oct 18;23(48):7990-8000. doi: 10.1038/sj.onc.1208074. Oncogene. 2004. PMID: 15489916 Review.
Cited by
-
G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA.PLoS Pathog. 2014 Jul 3;10(7):e1004242. doi: 10.1371/journal.ppat.1004242. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 24992036 Free PMC article.
-
Lipid raft targeting of hematopoietic protein tyrosine phosphatase by protein kinase C theta-mediated phosphorylation.Mol Cell Biol. 2006 Mar;26(5):1806-16. doi: 10.1128/MCB.26.5.1806-1816.2006. Mol Cell Biol. 2006. PMID: 16479000 Free PMC article.
-
Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue.J Biol Chem. 2010 Aug 20;285(34):26506-18. doi: 10.1074/jbc.M110.111104. Epub 2010 Jun 9. J Biol Chem. 2010. PMID: 20538612 Free PMC article.
-
Identification of c-Src tyrosine kinase substrates using mass spectrometry and peptide microarrays.J Proteome Res. 2008 Sep;7(9):3900-10. doi: 10.1021/pr800198w. Epub 2008 Aug 13. J Proteome Res. 2008. PMID: 18698806 Free PMC article.
-
An adaptor role for cytoplasmic Sam68 in modulating Src activity during cell polarization.Mol Cell Biol. 2009 Apr;29(7):1933-43. doi: 10.1128/MCB.01707-08. Epub 2009 Jan 12. Mol Cell Biol. 2009. PMID: 19139276 Free PMC article.
References
-
- Alonso, A., S. Rahmouni, S. Williams, M. van Stipdonk, L. Jaroszewski, A. Godzik, R. T. Abraham, S. P. Schoenberger, and T. Mustelin. 2003. Tyrosine phosphorylation of VHR by ZAP-70. Nat. Immunol. 4:44-48. - PubMed
-
- Barnes, C. J., F. Li, M. Mandal, Z. Yang, A. A. Sahin, and R. Kumar. 2002. Heregulin induces expression, ATPase activity, and nuclear localization of G3BP, a Ras signaling component, in human breast tumors. Cancer Res. 62:1251-1255. - PubMed
-
- Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adapter protein, binds the protein tyrosine kinase csk and is involved in regulation of T-cell activation. J. Exp. Med. 191:1591-1604. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
