Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4
- PMID: 15743827
- PMCID: PMC1061612
- DOI: 10.1128/MCB.25.6.2320-2330.2005
Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4
Abstract
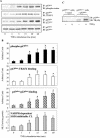
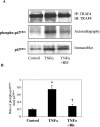
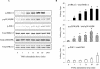
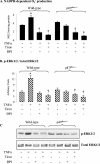
Tumor necrosis factor alpha (TNF-alpha) receptor-associated factors (TRAFs) play important roles in TNF-alpha signaling by interacting with downstream signaling molecules, e.g., mitogen-activated protein kinases (MAPKs). However, TNF-alpha also signals through reactive oxygen species (ROS)-dependent pathways. The interrelationship between these pathways is unclear; however, a recent study suggested that TRAF4 could bind to the NADPH oxidase subunit p47phox. Here, we investigated the potential interaction between p47phox phosphorylation and TRAF4 binding and their relative roles in acute TNF-alpha signaling. Exposure of human microvascular endothelial cells (HMEC-1) to TNF-alpha (100 U/ml; 1 to 60 min) induced rapid (within 5 min) p47phox phosphorylation. This was paralleled by a 2.7- +/- 0.5-fold increase in p47phox-TRAF4 association, membrane translocation of p47phox-TRAF4, a 2.3- +/- 0.4-fold increase in p47phox-p22phox complex formation, and a 3.2- +/- 0.2-fold increase in NADPH-dependent O2- production (all P < 0.05). TRAF4-p47phox binding was accompanied by a progressive increase in extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p38(MAPK) activation, which was inhibited by an O2- scavenger, tiron. TRAF4 predominantly bound the phosphorylated form of p47phox, in a protein kinase C-dependent process. Knockdown of TRAF4 expression using siRNA had no effect on p47phox phosphorylation or binding to p22phox but inhibited TNF-alpha-induced ERK1/2 activation. In coronary microvascular EC from p47phox-/- mice, TNF-alpha-induced NADPH oxidase activation, ERK1/2 activation, and cell surface intercellular adhesion molecule 1 (ICAM-1) expression were all inhibited. Thus, both p47phox phosphorylation and TRAF4 are required for acute TNF-alpha signaling. The increased binding between p47phox and TRAF4 that occurs after p47phox phosphorylation could serve to spatially confine ROS generation from NADPH oxidase and subsequent MAPK activation and cell surface ICAM-1 expression in EC.
Figures





Similar articles
-
Divergent effects of p47(phox) phosphorylation at S303-4 or S379 on tumor necrosis factor-α signaling via TRAF4 and MAPK in endothelial cells.Arterioscler Thromb Vasc Biol. 2012 Jun;32(6):1488-96. doi: 10.1161/ATVBAHA.112.247775. Epub 2012 Mar 29. Arterioscler Thromb Vasc Biol. 2012. PMID: 22460559
-
Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2- production, vascular tone, and mitogen-activated protein kinase activation.Circulation. 2004 Mar 16;109(10):1307-13. doi: 10.1161/01.CIR.0000118463.23388.B9. Epub 2004 Mar 1. Circulation. 2004. PMID: 14993144
-
Rac1 mediates sex difference in cardiac tumor necrosis factor-alpha expression via NADPH oxidase-ERK1/2/p38 MAPK pathway in endotoxemia.J Mol Cell Cardiol. 2009 Aug;47(2):264-74. doi: 10.1016/j.yjmcc.2009.05.002. Epub 2009 May 18. J Mol Cell Cardiol. 2009. PMID: 19450605
-
Localizing NADPH oxidase-derived ROS.Sci STKE. 2006 Aug 22;2006(349):re8. doi: 10.1126/stke.3492006re8. Sci STKE. 2006. PMID: 16926363 Review.
-
Role of NADPH oxidases in the control of vascular gene expression.Antioxid Redox Signal. 2003 Dec;5(6):803-11. doi: 10.1089/152308603770380115. Antioxid Redox Signal. 2003. PMID: 14588154 Review.
Cited by
-
Targeting the redox balance in inflammatory skin conditions.Int J Mol Sci. 2013 Apr 26;14(5):9126-67. doi: 10.3390/ijms14059126. Int J Mol Sci. 2013. PMID: 23624605 Free PMC article. Review.
-
NADPH oxidase-derived reactive oxygen species are involved in the HL-60 cell monocytic differentiation induced by isoliquiritigenin.Molecules. 2012 Nov 12;17(11):13424-38. doi: 10.3390/molecules171113424. Molecules. 2012. PMID: 23147401 Free PMC article.
-
Proliferative role of TRAF4 in breast cancer by upregulating PRMT5 nuclear expression.Tumour Biol. 2015 Aug;36(8):5901-11. doi: 10.1007/s13277-015-3262-0. Epub 2015 Feb 24. Tumour Biol. 2015. PMID: 25704480
-
Role for Traf4 in polarizing adherens junctions as a prerequisite for efficient cell shape changes.Mol Cell Biol. 2011 Dec;31(24):4978-93. doi: 10.1128/MCB.05542-11. Epub 2011 Oct 10. Mol Cell Biol. 2011. PMID: 21986496 Free PMC article.
-
Enhancement by tumor necrosis factor alpha of dengue virus-induced endothelial cell production of reactive nitrogen and oxygen species is key to hemorrhage development.J Virol. 2008 Dec;82(24):12312-24. doi: 10.1128/JVI.00968-08. Epub 2008 Oct 8. J Virol. 2008. PMID: 18842737 Free PMC article.
References
-
- Ades, E. W., F. J. Candal, R. A. G. V. G. Swerlick, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. - PubMed
-
- Aggarwal, B. B. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3:745-756. - PubMed
-
- Bayraktutan, U., L. Blayney, and A. M. Shah. 2000. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20:1903-1911. - PubMed
-
- Chandel, N. S., P. T. Schumacker, and R. H. Arch. 2001. Reactive oxygen species are downstream products of TRAF-mediated signaling transduction. J. Biol. Chem. 276:42728-42736. - PubMed
-
- Chen, X.-L., Q. Zhang, R. Zhao, X. Ding, P. E. Tummala, and R. M. Medford. 2003. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-α in endothelial cells. J. Pharmacol. Exp. Ther. 305:573-580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
