Activation of hematopoietic progenitor kinase 1 involves relocation, autophosphorylation, and transphosphorylation by protein kinase D1
- PMID: 15743830
- PMCID: PMC1061595
- DOI: 10.1128/MCB.25.6.2364-2383.2005
Activation of hematopoietic progenitor kinase 1 involves relocation, autophosphorylation, and transphosphorylation by protein kinase D1
Abstract
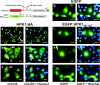
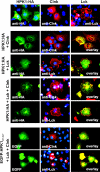
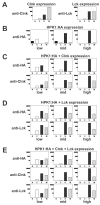
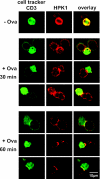
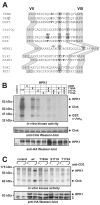
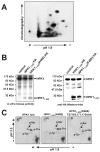
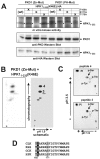
Adaptive immune signaling can be coupled to stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) and NF-kappaB activation by the hematopoietic progenitor kinase 1 (HPK1), a mammalian hematopoiesis-specific Ste20 kinase. To gain insight into the regulation of leukocyte signal transduction, we investigated the molecular details of HPK1 activation. Here we demonstrate the capacity of the Src family kinase Lck and the SLP-76 family adaptor protein Clnk (cytokine-dependent hematopoietic cell linker) to induce HPK1 tyrosine phosphorylation and relocation to the plasma membrane, which in lymphocytes results in recruitment of HPK1 to the contact site of antigen-presenting cell (APC)-T-cell conjugates. Relocation and clustering of HPK1 cause its enzymatic activation, which is accompanied by phosphorylation of regulatory sites in the HPK1 kinase activation loop. We show that full activation of HPK1 is dependent on autophosphorylation of threonine 165 and phosphorylation of serine 171, which is a target site for protein kinase D (PKD) in vitro. Upon T-cell receptor stimulation, PKD robustly augments HPK1 kinase activity in Jurkat T cells and enhances HPK1-driven SAPK/JNK and NF-kappaB activation; conversely, antisense down-regulation of PKD results in reduced HPK1 activity. Thus, activation of major lymphocyte signaling pathways via HPK1 involves (i) relocation, (ii) autophosphorylation, and (iii) transphosphorylation of HPK1 by PKD.
Figures


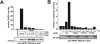


References
-
- Anafi, M., F. Kiefer, G. D. Gish, G. Mbamalu, N. N. Iscove, and T. Pawson. 1997. SH2/SH3 adaptor proteins can link tyrosine kinases to a Ste20-related protein kinase, HPK1. J. Biol. Chem. 272:27804-27811. - PubMed
-
- Arnold, R., J. Liou, H. C. Drexler, A. Weiss, and F. Kiefer. 2001. Caspase-mediated cleavage of hematopoietic progenitor kinase 1 (HPK1) converts an activator of NFκB into an inhibitor of NFκB. J. Biol. Chem. 276:14675-14684. - PubMed
-
- Biondi, R. M. 2004. Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem. Sci. 29:136-142. - PubMed
-
- Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
