The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression
- PMID: 15743831
- PMCID: PMC1061616
- DOI: 10.1128/MCB.25.6.2384-2394.2005
The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression
Abstract
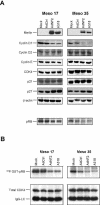
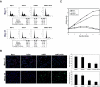
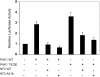
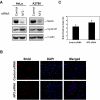
Inactivation of the NF2 tumor suppressor gene has been observed in certain benign and malignant tumors. Recent studies have demonstrated that merlin, the product of the NF2 gene, is regulated by Rac/PAK signaling. However, the mechanism by which merlin acts as a tumor suppressor has remained obscure. In this report, we show that adenovirus-mediated expression of merlin in NF2-deficient tumor cells inhibits cell proliferation and arrests cells at G1 phase, concomitant with decreased expression of cyclin D1, inhibition of CDK4 activity, and dephosphorylation of pRB. The effect of merlin on cell cycle progression was partially overridden by ectopic expression of cyclin D1. RNA interference experiments showed that silencing of the endogenous NF2 gene results in upregulation of cyclin D1 and S-phase entry. Furthermore, PAK1-stimulated cyclin D1 promoter activity was repressed by cotransfection of NF2, and PAK activity was inhibited by expression of merlin. Interestingly, the S518A mutant form of merlin, which is refractory to phosphorylation by PAK, was more efficient than the wild-type protein in inhibiting cell cycle progression and in repressing cyclin D1 promoter activity. Collectively, our data indicate that merlin exerts its antiproliferative effect, at least in part, via repression of PAK-induced cyclin D1 expression, suggesting a unifying mechanism by which merlin inactivation might contribute to the overgrowth seen in both noninvasive and malignant tumors.
Figures



References
-
- Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R. G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589-23597. - PubMed
-
- Balasenthil, S., A. A. Sahin, C. J. Barnes, R. A. Wang, R. G. Pestell, R. K. Vadlamudi, and R. Kumar. 2004. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J. Biol. Chem. 279:1422-1428. - PubMed
-
- Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. - PubMed
-
- Bianchi, A. B., S.-I. Mitsunaga, J. Q. Cheng, W. M. Klein, S. C. Jhanwar, B. Seizinger, N. Kley, A. J. P. Klein-Szanto, and J. R. Testa. 1995. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc. Natl. Acad. Sci. USA 92:10854-10858. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA075503/CA/NCI NIH HHS/United States
- R01 CA093596/CA/NCI NIH HHS/United States
- R01 CA045745/CA/NCI NIH HHS/United States
- R29 CA070896/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- T32-16850/PHS HHS/United States
- CA-45745/CA/NCI NIH HHS/United States
- CA-75503/CA/NCI NIH HHS/United States
- CA-93596/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
- CA-86072/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- CA-70896/CA/NCI NIH HHS/United States
- CA-06927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
