p21WAF1/CIP1 selectively controls the transcriptional activity of estrogen receptor alpha
- PMID: 15743834
- PMCID: PMC1061593
- DOI: 10.1128/MCB.25.6.2419-2430.2005
p21WAF1/CIP1 selectively controls the transcriptional activity of estrogen receptor alpha
Abstract
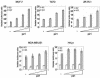
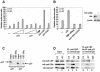
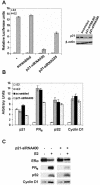
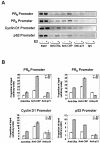
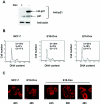
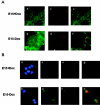
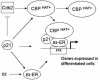
Estrogen receptors (ER) are ligand-dependent transcription factors that regulate growth, differentiation, and maintenance of cellular functions in a wide variety of tissues. We report here that p21WAF1/CIP1, a cyclin-dependent kinase (Cdk) inhibitor, cooperates with CBP to regulate the ERalpha-mediated transcription of endogenous target genes in a promoter-specific manner. The estrogen-induced expression of the progesterone receptor and WISP-2 mRNA transcripts in MCF-7 cells was enhanced by p21WAF1/CIP1, whereas that of the cyclin D1 mRNA was reduced and the pS2 mRNA was not affected. Chromatin immunoprecipitation assays revealed that p21WAF1/CIP1 was recruited simultaneously with ERalpha and CBP to the endogenous progesterone receptor gene promoter in an estrogen-dependent manner. Experiments in which the p21WAF1/CIP1 protein was knocked down by RNA interference showed that the induction of the expression of the gene encoding the progesterone receptor required p21WAF1/CIP1, in contrast with that of the cyclin D1 and pS2 genes. p21WAF1/CIP1 induced not only cell cycle arrest in breast cancer cells but also milk fat globule protein and lipid droplets, indicators of the differentiated phenotype, as well as cell flattening and increase of the volume of the cytoplasm. These results indicate that p21WAF1/CIP1, in addition to its Cdk-regulatory role, behaves as a transcriptional coactivator in a gene-specific manner implicated in cell differentiation.
Figures
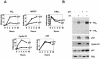
References
-
- Albanese, C., M. D'Amico, A. T. Reutens, M. Fu, G. Watanabe, R. J. Lee, R. N. Kitsis, B. Henglein, M. Avantaggiati, K. Somasundaram, B. Thimmapaya, and R. G. Pestell. 1999. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274:34186-34195. - PubMed
-
- Brouillard, F., and C. E. Cremisi. 2003. Concomitant increase of histone acetyltransferase activity and degradation of p300 during retinoic acid-induced differentiation of F9 cells. J. Biol. Chem. 278:39509-39516. - PubMed
-
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
