Vascular endothelial growth factor D is dispensable for development of the lymphatic system
- PMID: 15743836
- PMCID: PMC1061605
- DOI: 10.1128/MCB.25.6.2441-2449.2005
Vascular endothelial growth factor D is dispensable for development of the lymphatic system
Abstract
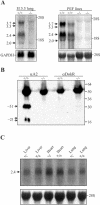
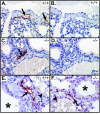
Vascular endothelial growth factor receptor 3 (Vegfr-3) is a tyrosine kinase that is expressed on the lymphatic endothelium and that signals for the growth of the lymphatic vessels (lymphangiogenesis). Vegf-d, a secreted glycoprotein, is one of two known activating ligands for Vegfr-3, the other being Vegf-c. Vegf-d stimulates lymphangiogenesis in tissues and tumors; however, its role in embryonic development was previously unknown. Here we report the generation and analysis of mutant mice deficient for Vegf-d. Vegf-d-deficient mice were healthy and fertile, had normal body mass, and displayed no pathologic changes consistent with a defect in lymphatic function. The lungs, sites of strong Vegf-d gene expression during embryogenesis in wild-type mice, were normal in Vegf-d-deficient mice with respect to tissue mass and morphology, except that the abundance of the lymphatics adjacent to bronchioles was slightly reduced. Dye uptake experiments indicated that large lymphatics under the skin were present in normal locations and were functional. Smaller dermal lymphatics were similar in number, location, and function to those in wild-type controls. The lack of a profound lymphatic phenotype in Vegf-d-deficient mice suggests that Vegf-d does not play a major role in lymphatic development or that Vegf-c or another, as-yet-unknown activating Vegfr-3 ligand can compensate for Vegf-d during development.
Figures



Similar articles
-
VEGF-D deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis.J Pathol. 2009 Nov;219(3):356-64. doi: 10.1002/path.2605. J Pathol. 2009. PMID: 19718705
-
Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice.EMBO J. 2001 Mar 15;20(6):1223-31. doi: 10.1093/emboj/20.6.1223. EMBO J. 2001. PMID: 11250889 Free PMC article.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats.Cancer Res. 2003 Feb 1;63(3):713-22. Cancer Res. 2003. PMID: 12566318
-
Tumor-induced lymphangiogenesis: a target for cancer therapy?J Biotechnol. 2006 Jun 25;124(1):224-41. doi: 10.1016/j.jbiotec.2006.01.007. Epub 2006 Feb 23. J Biotechnol. 2006. PMID: 16497404 Review.
Cited by
-
Blood vessels as targets in tumor therapy.Ups J Med Sci. 2012 May;117(2):178-86. doi: 10.3109/03009734.2012.660550. Epub 2012 Feb 21. Ups J Med Sci. 2012. PMID: 22348394 Free PMC article. Review.
-
Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD.J Clin Invest. 2016 Jun 1;126(6):2167-80. doi: 10.1172/JCI83967. Epub 2016 May 9. J Clin Invest. 2016. PMID: 27159393 Free PMC article.
-
EGFL7 loss correlates with increased VEGF-D expression, upregulating hippocampal adult neurogenesis and improving spatial learning and memory.Cell Mol Life Sci. 2023 Jan 30;80(2):54. doi: 10.1007/s00018-023-04685-z. Cell Mol Life Sci. 2023. PMID: 36715759 Free PMC article.
-
Emerging Roles for VEGF-D in Human Disease.Biomolecules. 2018 Jan 4;8(1):1. doi: 10.3390/biom8010001. Biomolecules. 2018. PMID: 29300337 Free PMC article. Review.
-
Lymphatic Vasculature in Energy Homeostasis and Obesity.Front Physiol. 2020 Jan 22;11:3. doi: 10.3389/fphys.2020.00003. eCollection 2020. Front Physiol. 2020. PMID: 32038308 Free PMC article. Review.
References
-
- Achen, M. G., S. Roufail, T. Domagala, B. Catimel, E. C. Nice, D. M. Geleick, R. Murphy, A. M. Scott, C. Caesar, T. Makinen, K. Alitalo, and S. A. Stacker. 2000. Monoclonal antibodies to vascular endothelial growth factor-D block interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem. 267:2505-2515. - PubMed
-
- Achen, M. G., R. A. Williams, M. E. Baldwin, P. Lai, S. Roufail, K. Alitalo, and S. A. Stacker. 2002. The angiogenic and lymphangiogenic factor vascular endothelial growth factor-D exhibits a paracrine mode of action in cancer. Growth Factors 20:99-107. - PubMed
-
- Achen, M. G., R. A. Williams, M. P. Minekus, G. E. Thornton, K. Stenvers, P. A. W. Rogers, F. Lederman, S. Roufail, and S. A. Stacker. 2001. Localization of vascular endothelial growth factor-D in malignant melanoma suggests a role in tumour angiogenesis. J. Pathol. 193:147-154. - PubMed
-
- Avantaggiato, V., M. Orlandini, D. Acampora, S. Oliviero, and A. Simeone. 1998. Embryonic expression pattern of the murine figf gene, a growth factor belonging to platelet-derived growth factor/vascular endothelial growth factor family. Mech. Dev. 73:221-224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
