Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions
- PMID: 15743837
- PMCID: PMC1061607
- DOI: 10.1128/MCB.25.6.2450-2462.2005
Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions
Abstract
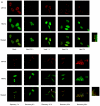
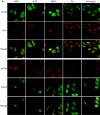
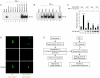
The cellular stress response (SR) is a phylogenetically conserved protection mechanism that involves inhibition of protein synthesis through recruitment of translation factors such as eIF4G into insoluble stress granules (SGs) and blockade of proinflammatory responses by interruption of the signaling pathway from tumor necrosis factor alpha (TNF-alpha) to nuclear factor-kappaB (NF-kappaB) activation. However, the link between these two physiological phenomena has not been clearly elucidated. Here we report that eIF4GI, which is a scaffold protein interacting with many translation factors, interacts with TRAF2, a signaling molecule that plays a key role in activation of NF-kappaB through TNF-alpha. These two proteins colocalize in SGs during cellular exposure to stress conditions. Moreover, TRAF2 is absent from TNFR1 complexes under stress conditions even after TNF-alpha treatment. This suggests that stressed cells lower their biological activities by sequestration of translation factors and TRAF2 into SGs through a protein-protein interaction.
Figures




References
-
- Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227-3234. - PubMed
-
- Arch, R. H., R. W. Gedrich, and C. B. Thompson. 2000. Translocation of TRAF proteins regulates apoptotic threshold of cells. Biochem. Biophys. Res. Commun. 272:936-945. - PubMed
-
- Back, S. H., J. E. Kim, J. Rho, B. Hahm, T. G. Lee, E. E. Kim, J. M. Cho, and S. K. Jang. 2000. Expression and purification of an active, full-length hepatitis C viral NS4A. Protein Expr. Purif. 20:196-206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
