FXYD3 (Mat-8), a new regulator of Na,K-ATPase
- PMID: 15743908
- PMCID: PMC1087241
- DOI: 10.1091/mbc.e04-10-0878
FXYD3 (Mat-8), a new regulator of Na,K-ATPase
Abstract
Four of the seven members of the FXYD protein family have been identified as specific regulators of Na,K-ATPase. In this study, we show that FXYD3, also known as Mat-8, is able to associate with and to modify the transport properties of Na,K-ATPase. In addition to this shared function, FXYD3 displays some uncommon characteristics. First, in contrast to other FXYD proteins, which were shown to be type I membrane proteins, FXYD3 may have a second transmembrane-like domain because of the presence of a noncleavable signal peptide. Second, FXYD3 can associate with Na,K- as well as H,K-ATPases when expressed in Xenopus oocytes. However, in situ (stomach), FXYD3 is associated only with Na,K-ATPase because its expression is restricted to mucous cells in which H,K-ATPase is absent. Coexpressed in Xenopus oocytes, FXYD3 modulates the glycosylation processing of the beta subunit of X,K-ATPase dependent on the presence of the signal peptide. Finally, FXYD3 decreases both the apparent affinity for Na+ and K+ of Na,K-ATPase.
Figures

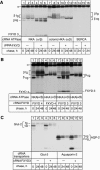


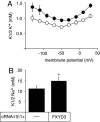
References
-
- Arystarkhova, E., Wetzel, R. K., Asinovski, N. K., and Sweadner, K. J. (1999). The γ subunit modulates Na+ and K+ affinity of the renal Na,K-ATPase. J. Biol. Chem. 274, 33183–33185. - PubMed
-
- Bendtsen, J. D., Nielsen, H., von Heijne, G., and Brunak, S. (2004). Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

