Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization
- PMID: 15743918
- PMCID: PMC555983
- DOI: 10.1073/pnas.0408536102
Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization
Abstract
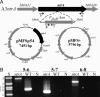
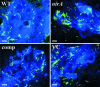
Previous studies have shown that a sigma54-sigma(S) cascade regulates the expression of a few key lipoproteins in Borrelia burgdorferi, the agent of Lyme disease. Here, we demonstrate that these sigma factors, both together and independently, regulate a much more extensive number of genes and cellular processes. Microarray analyses of sigma54 and sigma(S) mutant strains identified 305 genes regulated by sigma54 and 145 regulated by sigma(S), whereas the sigma54-sigma(S) regulatory cascade appears to control 48 genes in B. burgdorferi. In silico analyses revealed that nearly 80% of genes with altered expression in the sigma54 mutant were linked to potential sigma54-dependent promoters. Many sigma54-regulated genes are expressed in vivo, and through genetic complementation of the mutant, we demonstrated that sigma54 was required by B. burgdorferi to infect mammals. Surprisingly, sigma54 mutants were able to infect Ixodes scapularis ticks and be maintained for at least 24 wk after infection, suggesting the sigma54-sigma(S) regulatory network was not involved in long-term survival in ticks. However, sigma54 mutants did not enter the salivary glands during tick feeding, indicating that sigma54-regulated genes were involved in the transmission process.
Figures



Comment in
-
Sigma cascades in prokaryotic regulatory networks.Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):4933-4. doi: 10.1073/pnas.0501417102. Epub 2005 Mar 28. Proc Natl Acad Sci U S A. 2005. PMID: 15795367 Free PMC article. No abstract available.
References
-
- Fraser, C. M., Casjens, S., Huang, W. M., Sutton, G. G., Clayton, R., Lathigra, R., White, O., Ketchum, K. A., Dodson, R., Hickey, E. K., et al. (1997) Nature 390, 580–586. - PubMed
-
- Casjens, S., Palmer, N., van Vugt, R., Huwang, W. M., Stevenson, B., Rosa, P., Lathigra, R., Sutton, G. G., Peterson, J., Dodson, R., et al. (2000) Mol. Microbiol. 35, 490–516. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

