Cytochrome bd oxidase, oxidative stress, and dioxygen tolerance of the strictly anaerobic bacterium Moorella thermoacetica
- PMID: 15743950
- PMCID: PMC1064043
- DOI: 10.1128/JB.187.6.2020-2029.2005
Cytochrome bd oxidase, oxidative stress, and dioxygen tolerance of the strictly anaerobic bacterium Moorella thermoacetica
Abstract
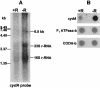
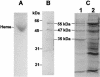
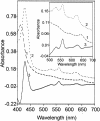
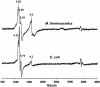
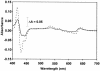
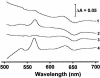
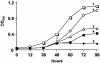
The gram-positive, thermophilic, acetogenic bacterium Moorella thermoacetica can reduce CO2 to acetate via the Wood-Ljungdahl (acetyl coenzyme A synthesis) pathway. This report demonstrates that, despite its classification as a strict anaerobe, M. thermoacetica contains a membrane-bound cytochrome bd oxidase that can catalyze reduction of low levels of dioxygen. Whole-cell suspensions of M. thermoacetica had significant endogenous O2 uptake activity, and this activity was increased in the presence of methanol or CO, which are substrates in the Wood-Ljungdahl pathway. Cyanide and azide strongly (approximately 70%) inhibited both the endogenous and CO/methanol-dependent O2 uptake. UV-visible light absorption and electron paramagnetic resonance spectra of n-dodecyl-beta-maltoside extracts of M. thermoacetica membranes showed the presence of a cytochrome bd oxidase complex containing cytochrome b561, cytochrome b595, and cytochrome d (chlorin). Subunits I and II of the bd oxidase were identified by N-terminal amino acid sequencing. The M. thermoacetica cytochrome bd oxidase exhibited cyanide-sensitive quinol oxidase activity. The M. thermoacetica cytochrome bd (cyd) operon consists of four genes, encoding subunits I and II along with two ABC-type transporter proteins, homologs of which in other bacteria are required for assembly of the bd complex. The level of this cyd operon transcript was significantly increased when M. thermoacetica was grown in the absence of added reducing agent (cysteine + H2S). Expression of a 35-kDa cytosolic protein, identified as a cysteine synthase (CysK), was also induced by the nonreducing growth conditions. The combined evidence indicates that cytochrome bd oxidase and cysteine synthase protect against oxidative stress and contribute to the limited dioxygen tolerance of M. thermoacetica.
Figures


Similar articles
-
Cyanide-insensitive quinol oxidase (CIO) from Gluconobacter oxydans is a unique terminal oxidase subfamily of cytochrome bd.J Biochem. 2013 Jun;153(6):535-45. doi: 10.1093/jb/mvt019. Epub 2013 Mar 22. J Biochem. 2013. PMID: 23526305
-
Characterization of a corrinoid protein involved in the C1 metabolism of strict anaerobic bacterium Moorella thermoacetica.Proteins. 2007 Apr 1;67(1):167-76. doi: 10.1002/prot.21094. Proteins. 2007. PMID: 17211893
-
Resonance Raman spectroscopic identification of a histidine ligand of b595 and the nature of the ligation of chlorin d in the fully reduced Escherichia coli cytochrome bd oxidase.Biochemistry. 1996 Feb 20;35(7):2403-12. doi: 10.1021/bi9518252. Biochemistry. 1996. PMID: 8652583
-
Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress.Biochim Biophys Acta. 2014 Jul;1837(7):1178-87. doi: 10.1016/j.bbabio.2014.01.016. Epub 2014 Jan 31. Biochim Biophys Acta. 2014. PMID: 24486503 Review.
-
The respiratory chain of Corynebacterium glutamicum.J Biotechnol. 2003 Sep 4;104(1-3):129-53. doi: 10.1016/s0168-1656(03)00144-5. J Biotechnol. 2003. PMID: 12948635 Review.
Cited by
-
Aerobic Microbial Respiration In Oceanic Oxygen Minimum Zones.PLoS One. 2015 Jul 20;10(7):e0133526. doi: 10.1371/journal.pone.0133526. eCollection 2015. PLoS One. 2015. PMID: 26192623 Free PMC article.
-
Stabilization of the Highly Hydrophobic Membrane Protein, Cytochrome bd Oxidase, on Metallic Surfaces for Direct Electrochemical Studies.Molecules. 2020 Jul 16;25(14):3240. doi: 10.3390/molecules25143240. Molecules. 2020. PMID: 32708635 Free PMC article.
-
Pathways of Iron and Sulfur Acquisition, Cofactor Assembly, Destination, and Storage in Diverse Archaeal Methanogens and Alkanotrophs.J Bacteriol. 2021 Aug 9;203(17):e0011721. doi: 10.1128/JB.00117-21. Epub 2021 Aug 9. J Bacteriol. 2021. PMID: 34124941 Free PMC article.
-
Quantitative RNA-seq Analysis Unveils Osmotic and Thermal Adaptation Mechanisms Relevant for Ectoine Production in Chromohalobacter salexigens.Front Microbiol. 2018 Aug 13;9:1845. doi: 10.3389/fmicb.2018.01845. eCollection 2018. Front Microbiol. 2018. PMID: 30158907 Free PMC article.
-
The cytochrome bd respiratory oxygen reductases.Biochim Biophys Acta. 2011 Nov;1807(11):1398-413. doi: 10.1016/j.bbabio.2011.06.016. Epub 2011 Jul 1. Biochim Biophys Acta. 2011. PMID: 21756872 Free PMC article. Review.
References
-
- Barnes, E. M., and M. Ingram. 1956. The effect of redox potential on the growth of Clostridium welchii strains isolated from horse muscle. J. Appl. Bacteriol. 19:117-122.
-
- Baughn, A. D., and M. N. Malamy. 2004. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427:441-444. - PubMed
-
- Beinert, H. 2000. Iron-sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5:2-15. - PubMed
-
- Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3:152-157. - PubMed
-
- Benov, L., N. M. Kredich, and I. Fridovich. 1996. The mechanism of the auxotrophy for sulfur-containing amino acids imposed upon Escherichia coli by superoxide. J. Biol. Chem. 271:21037-21040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

