Cyclic AMP (cAMP) and cAMP receptor protein influence both synthesis and uptake of extracellular autoinducer 2 in Escherichia coli
- PMID: 15743955
- PMCID: PMC1064054
- DOI: 10.1128/JB.187.6.2066-2076.2005
Cyclic AMP (cAMP) and cAMP receptor protein influence both synthesis and uptake of extracellular autoinducer 2 in Escherichia coli
Abstract
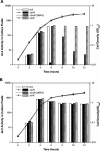
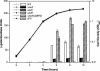
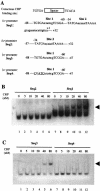
Bacterial autoinducer 2 (AI-2) is proposed to be an interspecies mediator of cell-cell communication that enables cells to operate at the multicellular level. Many environmental stimuli have been shown to affect the extracellular AI-2 levels, carbon sources being among the most important. In this report, we show that both AI-2 synthesis and uptake in Escherichia coli are subject to catabolite repression through the cyclic AMP (cAMP)-CRP complex, which directly stimulates transcription of the lsr (for "luxS regulated") operon and indirectly represses luxS expression. Specifically, cAMP-CRP is shown to bind to a CRP binding site located in the upstream region of the lsr promoter and works with the LsrR repressor to regulate AI-2 uptake. The functions of the lsr operon and its regulators, LsrR and LsrK, previously reported in Salmonella enterica serovar Typhimurium, are confirmed here for E. coli. The elucidation of cAMP-CRP involvement in E. coli autoinduction impacts many areas, including the growth of E. coli in fermentation processes.
Figures

References
-
- Ahmer, B. M. 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol. Microbiol. 52:933-945. - PubMed
-
- Bainton, N. J., B. W. Bycroft, S. R. Chhabra, P. Stead, L. Gledhill, P. J. Hill, C. E. Rees, M. K. Winson, G. P. Salmond, G. S. Stewart, et al. 1992. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene 116:87-91. - PubMed
-
- Barber, A. M., and V. B. Zhurkin. 1990. CAP binding sites reveal pyrimidine-purine pattern characteristic of DNA bending. J. Biomol. Struct. Dyn. 8:213-232. - PubMed
-
- Barber, A. M., V. B. Zhurkin, and S. Adhya. 1993. CRP-binding sites: evidence for two structural classes with 6-bp and 8-bp spacers. Gene 130:1-8. - PubMed
-
- Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

