Characterization of a novel zinc-containing, lysine-specific aminopeptidase from the hyperthermophilic archaeon Pyrococcus furiosus
- PMID: 15743956
- PMCID: PMC1064047
- DOI: 10.1128/JB.187.6.2077-2083.2005
Characterization of a novel zinc-containing, lysine-specific aminopeptidase from the hyperthermophilic archaeon Pyrococcus furiosus
Abstract
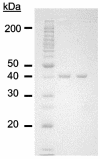
Cell extracts of the proteolytic, hyperthermophilic archaeon Pyrococcus furiosus contain high specific activity (11 U/mg) of lysine aminopeptidase (KAP), as measured by the hydrolysis of L-lysyl-p-nitroanilide (Lys-pNA). The enzyme was purified by multistep chromatography. KAP is a homotetramer (38.2 kDa per subunit) and, as purified, contains 2.0 +/- 0.48 zinc atoms per subunit. Surprisingly, its activity was stimulated fourfold by the addition of Co2+ ions (0.2 mM). Optimal KAP activity with Lys-pNA as the substrate occurred at pH 8.0 and a temperature of 100 degrees C. The enzyme had a narrow substrate specificity with di-, tri-, and tetrapeptides, and it hydrolyzed only basic N-terminal residues at high rates. Mass spectroscopy analysis of the purified enzyme was used to identify, in the P. furiosus genome database, a gene (PF1861) that encodes a product corresponding to 346 amino acids. The recombinant protein containing a polyhistidine tag at the N terminus was produced in Escherichia coli and purified using affinity chromatography. Its properties, including molecular mass, metal ion dependence, and pH and temperature optima for catalysis, were indistinguishable from those of the native form, although the thermostability of the recombinant form was dramatically lower than that of the native enzyme (half-life of approximately 6 h at 100 degrees C). Based on its amino acid sequence, KAP is part of the M18 family of peptidases and represents the first prokaryotic member of this family. KAP is also the first lysine-specific aminopeptidase to be purified from an archaeon.
Figures


Similar articles
-
Characterization of native and recombinant forms of an unusual cobalt-dependent proline dipeptidase (prolidase) from the hyperthermophilic archaeon Pyrococcus furiosus.J Bacteriol. 1998 Sep;180(18):4781-9. doi: 10.1128/JB.180.18.4781-4789.1998. J Bacteriol. 1998. PMID: 9733678 Free PMC article.
-
Characterization of an aminoacylase from the hyperthermophilic archaeon Pyrococcus furiosus.J Bacteriol. 2001 Jul;183(14):4259-68. doi: 10.1128/JB.183.14.4259-4268.2001. J Bacteriol. 2001. PMID: 11418567 Free PMC article.
-
Methionine aminopeptidase from the hyperthermophilic Archaeon Pyrococcus furiosus: molecular cloning and overexpression in Escherichia coli of the gene, and characteristics of the enzyme.J Biochem. 1997 Oct;122(4):843-50. doi: 10.1093/oxfordjournals.jbchem.a021831. J Biochem. 1997. PMID: 9399590
-
The Development of Tungsten Biochemistry-A Personal Recollection.Molecules. 2023 May 11;28(10):4017. doi: 10.3390/molecules28104017. Molecules. 2023. PMID: 37241758 Free PMC article. Review.
-
Molecular advances in microbial aminopeptidases.Bioresour Technol. 2017 Dec;245(Pt B):1757-1765. doi: 10.1016/j.biortech.2017.05.103. Epub 2017 May 19. Bioresour Technol. 2017. PMID: 28599921 Review.
Cited by
-
A novel aminopeptidase with highest preference for lysine.Neurochem Res. 2006 Jan;31(1):95-102. doi: 10.1007/s11064-005-9234-9. Neurochem Res. 2006. PMID: 16475002
-
A computational framework for proteome-wide pursuit and prediction of metalloproteins using ICP-MS and MS/MS data.BMC Bioinformatics. 2011 Feb 28;12:64. doi: 10.1186/1471-2105-12-64. BMC Bioinformatics. 2011. PMID: 21356119 Free PMC article.
-
Tuned by metals: the TET peptidase activity is controlled by 3 metal binding sites.Sci Rep. 2016 Feb 8;6:20876. doi: 10.1038/srep20876. Sci Rep. 2016. PMID: 26853450 Free PMC article.
-
Metallo-aminopeptidase inhibitors.Biochimie. 2010 Nov;92(11):1509-29. doi: 10.1016/j.biochi.2010.04.026. Epub 2010 May 10. Biochimie. 2010. PMID: 20457213 Free PMC article. Review.
-
Transcriptional and biochemical analysis of starch metabolism in the hyperthermophilic archaeon Pyrococcus furiosus.J Bacteriol. 2006 Mar;188(6):2115-25. doi: 10.1128/JB.188.6.2115-2125.2006. J Bacteriol. 2006. PMID: 16513741 Free PMC article.
References
-
- Adams, M. W. 1999. The biochemical diversity of life near and above 100°C in marine environments. J. Appl. Microbiol. Symp. Suppl. 85:108S. - PubMed
-
- Adams, M. W., and A. Kletzin. 1996. Oxidoreductase-type enzymes and redox proteins involved in fermentative metabolisms of hyperthermophilic Archaea. Adv. Protein Chem. 48:101-180. - PubMed
-
- Ando, S., K. Ishikawa, H. Ishida, Y. Kawarabayasi, H. Kikuchi, and Y. Kosugi. 1999. Thermostable aminopeptidase from Pyrococcus horikoshii. FEBS Lett. 447:25-28. - PubMed
-
- Andreotti, G., M. V. Cubellis, G. Nitti, G. Sannia, X. Mai, M. W. Adams, and G. Marino. 1995. An extremely thermostable aromatic aminotransferase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim. Biophys. Acta 1247:90-96. - PubMed
-
- Arfin, S. M., and R. A. Bradshaw. 1988. Cotranslational processing and protein turnover in eukaryotic cells. Biochemistry 27:7979-7984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

