Phosphatidylethanolamine domains and localization of phospholipid synthases in Bacillus subtilis membranes
- PMID: 15743965
- PMCID: PMC1064036
- DOI: 10.1128/JB.187.6.2163-2174.2005
Phosphatidylethanolamine domains and localization of phospholipid synthases in Bacillus subtilis membranes
Abstract
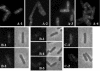
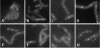
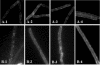
Application of the cardiolipin (CL)-specific fluorescent dye 10-N-nonyl-acridine orange has recently revealed CL-rich domains in the septal regions and at the poles of the Bacillus subtilis membrane (F. Kawai, M. Shoda, R. Harashima, Y. Sadaie, H. Hara, and K. Matsumoto, J. Bacteriol. 186:1475-1483, 2004). This finding prompted us to examine the localization of another phospholipid, phosphatidylethanolamine (PE), with the cyclic peptide probe, Ro09-0198 (Ro), that binds specifically to PE. Treatment with biotinylated Ro followed by tetramethyl rhodamine-conjugated streptavidin revealed that PE is localized in the septal membranes of vegetative cells and in the membranes of the polar septum and the engulfment membranes of sporulating cells. When the mutant cells of the strains SDB01 (psd1::neo) and SDB02 (pssA10::spc), which both lack PE, were examined under the same conditions, no fluorescence was observed. The localization of the fluorescence thus evidently reflected the localization of PE-rich domains in the septal membranes. Similar PE-rich domains were observed in the septal regions of the cells of many Bacillus species. In Escherichia coli cells, however, no PE-rich domains were found. Green fluorescent protein fusions to the enzymes that catalyze the committed steps in PE synthesis, phosphatidylserine synthase, and in CL synthesis, CL synthase and phosphatidylglycerophosphate synthase, were localized mainly in the septal membranes in B. subtilis cells. The majority of the lipid synthases were also localized in the septal membranes; this includes 1-acyl-glycerol-3-phosphate acyltransferase, CDP-diacylglycerol synthase, phosphatidylserine decarboxylase, diacylglycerol kinase, glucolipid synthase, and lysylphosphatidylglycerol synthase. These results suggest that phospholipids are produced mostly in the septal membranes and that CL and PE are kept from diffusing out to lateral ones.
Figures



Similar articles
-
A regulatory mechanism for the balanced synthesis of membrane phospholipid species in Escherichia coli.Biosci Biotechnol Biochem. 1996 Jan;60(1):111-6. doi: 10.1271/bbb.60.111. Biosci Biotechnol Biochem. 1996. PMID: 8824831
-
Cardiolipin domains in Bacillus subtilis marburg membranes.J Bacteriol. 2004 Mar;186(5):1475-83. doi: 10.1128/JB.186.5.1475-1483.2004. J Bacteriol. 2004. PMID: 14973018 Free PMC article.
-
Septal localization by membrane targeting sequences and a conserved sequence essential for activity at the COOH-terminus of Bacillus subtilis cardiolipin synthase.Res Microbiol. 2016 Apr;167(3):202-14. doi: 10.1016/j.resmic.2015.11.004. Epub 2015 Dec 18. Res Microbiol. 2016. PMID: 26708983
-
Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells.Biochim Biophys Acta. 2013 Mar;1831(3):543-54. doi: 10.1016/j.bbalip.2012.08.016. Epub 2012 Aug 29. Biochim Biophys Acta. 2013. PMID: 22960354 Review.
-
Lipid domains in bacterial membranes.Mol Microbiol. 2006 Sep;61(5):1110-7. doi: 10.1111/j.1365-2958.2006.05317.x. Mol Microbiol. 2006. PMID: 16925550 Review.
Cited by
-
The milk fat globule size governs a physiological switch for biofilm formation by Bacillus subtilis.Front Nutr. 2022 Aug 11;9:844587. doi: 10.3389/fnut.2022.844587. eCollection 2022. Front Nutr. 2022. PMID: 36034896 Free PMC article.
-
RodZ and PgsA Play Intertwined Roles in Membrane Homeostasis of Bacillus subtilis and Resistance to Weak Organic Acid Stress.Front Microbiol. 2016 Oct 21;7:1633. doi: 10.3389/fmicb.2016.01633. eCollection 2016. Front Microbiol. 2016. PMID: 27818647 Free PMC article.
-
Differential actions of chlorhexidine on the cell wall of Bacillus subtilis and Escherichia coli.PLoS One. 2012;7(5):e36659. doi: 10.1371/journal.pone.0036659. Epub 2012 May 11. PLoS One. 2012. PMID: 22606280 Free PMC article.
-
The MprF homolog LysX synthesizes lysyl-diacylglycerol contributing to antibiotic resistance and virulence.Microbiol Spectr. 2023 Sep 28;11(5):e0142923. doi: 10.1128/spectrum.01429-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37768052 Free PMC article.
-
Cardiolipin membrane domains in prokaryotes and eukaryotes.Biochim Biophys Acta. 2009 Oct;1788(10):2084-91. doi: 10.1016/j.bbamem.2009.04.003. Epub 2009 Apr 14. Biochim Biophys Acta. 2009. PMID: 19371718 Free PMC article. Review.
References
-
- Christensen, H., N. J. Garton, R. W. Horobin, D. E. Minnikin, and M. R. Barer. 1999. Lipid domains of mycobacteria studied with fluorescent molecular probes. Mol. Microbiol. 31:1561-1572. - PubMed
-
- Cole, R., and P. Proulx. 1977. Further studies on the cardiolipin phosphodiesterase of Escherichia coli. Can. J. Biochem. 55:1228-1232. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

