Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response
- PMID: 15744129
- PMCID: PMC2253680
- DOI: 10.1089/sur.2004.5.375
Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response
Abstract
Background: Sepsis remains a substantial risk after surgery or other trauma. Macrophage dysfunction, as a component of immune suppression seen during trauma and sepsis, appears to be one of the contributing factors to morbidity and mortality. However, whereas it is known that the ability of macrophages to present antigen and express major histocompatibility complex MHC class II molecules is decreased during sepsis, it is not known to what extent this is associated with the loss of co-stimulatory receptor expression. Our objectives in this study were, therefore, to determine if the expression of co-stimulatory molecules, such as CD40, CD80, or CD86, on peritoneal/splenic/liver macrophages were altered by sepsis (cecal ligation [CL] and puncture [CLP] or necrotic tissue injury (CL) alone; and to establish the contribution of such changes to the response to septic challenge using mice that are deficient in these receptors.
Methods: To address our first objective, male C3H/HeN mice were subjected to CLP, CL, or sham (n = four to six mice/group), and the adherent macrophages were isolated from the peritoneum, spleen, or liver at 24 h post-insult. The macrophages were then analyzed by flow cytometry for their ex vivo expression of CD40, CD80, CD86, and/or MHC II.
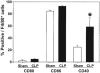
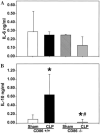
Results: The expression of CD86 and MHC II, but not CD40 or CD80, were significantly decreased on peritoneal macrophages after the onset of sepsis or CL alone. In addition, CD40 expression was significantly increased in Kupffer cells after sepsis. Alternatively, splenic macrophages from septic or CL mice did not show changes in the expression of CD80, CD86, or CD40. To the degree that the loss of CD86 expression might contribute to the changes reported in macrophage function in septic mice, we subsequently examined the effects of CLP on CD86 -/- mice. Interestingly, we found that, unlike the background controls, neither the serum IL-10 concentrations nor the IL-10 release capacity of peritoneal macrophages from septic CD86 -/- mice were increased.
Conclusion: Together, these data suggest a potential role for the co-stimulatory receptor CD86/B7-2 beyond that of simply promoting competent antigen presentation to T-cells, but also as a regulator of the anti-inflammatory IL-10 response. Such a role may implicate the latter response in the development of sepsis-induced immune dysfunction.
Figures




References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. - PubMed
-
- Lanier LL, O’Fallon S, Somoza C, et al. CD80 (B7-1) and CD86 (B7-2) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

