Trafficking of a ligand-receptor complex on the growth cones as an essential step for the uptake of nerve growth factor at the distal end of the axon: a single-molecule analysis
- PMID: 15745944
- PMCID: PMC6726083
- DOI: 10.1523/JNEUROSCI.4570-04.2005
Trafficking of a ligand-receptor complex on the growth cones as an essential step for the uptake of nerve growth factor at the distal end of the axon: a single-molecule analysis
Abstract
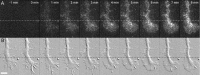
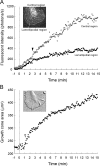
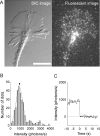
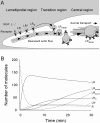
The behavior of single molecules of neurotrophins on growth cones was observed by the use of the fluorescent conjugate of nerve growth factor (NGF), Cy3-NGF. After the application of 0.4 nm Cy3-NGF, chick dorsal root ganglion growth cones responded within 1 min of adding the stimulus by expanding their lamellipodia. Only 40 molecules of Cy3-NGF, which occupied <5% of the estimated total binding sites on a single growth cone, were required to initiate the motile responses. After binding to the high-affinity receptor, Cy3-NGF displayed lateral diffusion on the membrane of the growth cones with a diffusion constant of 0.3 microm2 s(-1). The behavior of Cy3-NGF was shifted to a one-directional rearward movement toward the central region of the growth cone. The one-directional movement of Cy3-NGF displayed the same rate as the rearward flow of actin, approximately 4 microm/min. This movement could be stopped by the application of the potent inhibitor of actin polymerization, latrunculin B. Molecules of Cy3-NGF were suggested to be internalized in the vicinity of the central region of the growth cone during this rearward trafficking, because Cy3-NGF remained in the growth cone after the growth cones had been exposed to an acidic surrounding medium: acidic medium causes the complete dissociation of Cy3-NGF from the receptors on the surface of growth cones. These results suggested that actin-driven trafficking of the NGF receptor complex is an essential step for the accumulation and endocytosis of NGF at the growth cone and for the retrograde transport of NGF toward the cell body.
Figures





References
-
- Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR, Sehgal A (1986) Gene transfer and molecular cloning of the human NGF receptor. Science 232: 518-521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
