Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes
- PMID: 15745945
- PMCID: PMC6726085
- DOI: 10.1523/JNEUROSCI.3965-04.2005
Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes
Erratum in
- J Neurosci. 2005 Mar 16;25(11):3022
Abstract
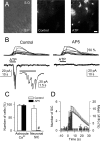
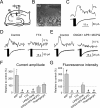
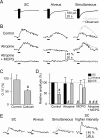
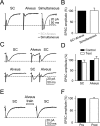
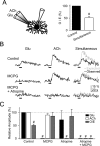
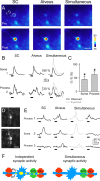
The synaptic control of the astrocytic intracellular Ca2+ is crucial in the reciprocal astrocyte-neuron communication. Using electrophysiological and Ca2+ imaging techniques in rat hippocampal slices, we investigated the astrocytic Ca2+ signal modulation induced by synaptic terminals that use glutamate and acetylcholine. Ca2+ elevations were evoked by glutamate released from Schaffer collaterals and by acetylcholine, but not glutamate, released by alveus stimulation, indicating that astrocytes discriminate the activity of different synapses belonging to different axon pathways. The Ca2+ signal was modulated bidirectionally by simultaneous activation of both pathways, being depressed at high stimulation frequencies and enhanced at low frequencies. The Ca2+ modulation was attributable to astrocytic intrinsic properties, occurred at discrete regions of the processes, and controlled the intracellular expansion of the Ca2+ signal. In turn, astrocyte Ca2+ signal elicited NMDA receptor-mediated currents in pyramidal neurons. Therefore, because astrocytes discriminate and integrate synaptic information, we propose that they can be considered as cellular elements involved in the information processing by the nervous system.
Figures


References
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1998a) Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci 10: 2129-2142. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208-215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
