Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation
- PMID: 15745948
- PMCID: PMC6726080
- DOI: 10.1523/JNEUROSCI.5065-04.2005
Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation
Abstract
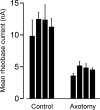
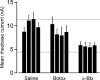
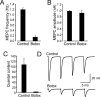
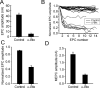
Motoneuron populations possess a range of intrinsic excitability that plays an important role in establishing how motor units are recruited. The fact that this range collapses after axotomy and does not recover completely until after reinnervation occurs suggests that muscle innervation is needed to maintain or regulate adult motoneuron excitability, but the nature and identity of underlying mechanisms remain poorly understood. Here, we report the results of experiments in which we studied the effects on rat motoneuron excitability produced by manipulations of neuromuscular transmission and compared these with the effects of peripheral nerve axotomy. Inhibition of acetylcholine release from motor terminals for 5-6 d with botulinum toxin produced relatively minor changes in motoneuron excitability compared with the effect of axotomy. In contrast, the blockade of acetylcholine receptors with alpha-bungarotoxin over the same time interval produced changes in motoneuron excitability that were statistically equivalent to axotomy. Muscle fiber recordings showed that low levels of acetylcholine release persisted at motor terminals after botulinum toxin, but endplate currents were completely blocked for at least several hours after daily intramuscular injections of alpha-bungarotoxin. We conclude that the complete but transient blockade of endplate currents underlies the robust axotomy-like effects of alpha-bungarotoxin on motoneuron excitability, and the low level of acetylcholine release that remains after injections of botulinum toxin inhibits axotomy-like changes in motoneurons. The results suggest the existence of a retrograde signaling mechanism located at the motor endplate that enables expression of adult motoneuron excitability and depends on acetylcholine receptor activation for its normal operation.
Figures
Similar articles
-
Rat motoneuron properties recover following reinnervation in the absence of muscle activity and evoked acetylcholine release.J Physiol. 2007 Nov 15;585(Pt 1):47-56. doi: 10.1113/jphysiol.2007.135541. Epub 2007 Sep 20. J Physiol. 2007. PMID: 17884931 Free PMC article.
-
Axotomy-like changes in cat motoneuron electrical properties elicited by botulinum toxin depend on the complete elimination of neuromuscular transmission.J Neurosci. 1991 Mar;11(3):657-66. doi: 10.1523/JNEUROSCI.11-03-00657.1991. J Neurosci. 1991. PMID: 1848281 Free PMC article.
-
Postsynaptic transmission block can cause terminal sprouting of a motor nerve.Science. 1980 Feb 8;207(4431):649-51. doi: 10.1126/science.6243417. Science. 1980. PMID: 6243417
-
[A reminder of the structure and function of the skeletal neuromuscular junction].Ann Dermatol Venereol. 2009 May;136 Suppl 4:S55-60. doi: 10.1016/S0151-9638(09)74528-4. Ann Dermatol Venereol. 2009. PMID: 19576486 Review. French.
-
Giant miniature endplate potentials at vertebrate neuromuscular junctions: A review.Eur J Neurosci. 2024 Dec;60(12):7183-7194. doi: 10.1111/ejn.16624. Epub 2024 Nov 26. Eur J Neurosci. 2024. PMID: 39600045 Review.
Cited by
-
The neurite outgrowth inhibitor Nogo-A promotes denervation in an amyotrophic lateral sclerosis model.EMBO Rep. 2006 Nov;7(11):1162-7. doi: 10.1038/sj.embor.7400826. Epub 2006 Oct 13. EMBO Rep. 2006. PMID: 17039253 Free PMC article.
-
Spinal cord from body donors is suitable for multicolor immunofluorescence.Histochem Cell Biol. 2023 Jan;159(1):23-45. doi: 10.1007/s00418-022-02154-5. Epub 2022 Oct 6. Histochem Cell Biol. 2023. PMID: 36201037 Free PMC article.
-
Neuromuscular dysfunction, independent of gait dysfunction, modulates trabecular bone homeostasis in mice.J Musculoskelet Neuronal Interact. 2019 Mar 1;19(1):79-93. J Musculoskelet Neuronal Interact. 2019. PMID: 30839306 Free PMC article.
-
Delaying the onset of treadmill exercise following peripheral nerve injury has different effects on axon regeneration and motoneuron synaptic plasticity.J Neurophysiol. 2015 Apr 1;113(7):2390-9. doi: 10.1152/jn.00892.2014. Epub 2015 Jan 28. J Neurophysiol. 2015. PMID: 25632080 Free PMC article.
-
Activity-dependent regulation of the binomial parameters p and n at the mouse neuromuscular junction in vivo.J Neurophysiol. 2010 Nov;104(5):2352-8. doi: 10.1152/jn.00460.2010. Epub 2010 Aug 25. J Neurophysiol. 2010. PMID: 20739593 Free PMC article.
References
-
- Binder MD, Powers RK (2001) Relationship between simulated common synaptic input and discharge synchrony in cat spinal motoneurons. J Neurophysiol 86: 2266-2275. - PubMed
-
- Burgess RW, Nguyen QT, Son YJ, Lichtman JW, Sanes JR (1999) Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron 23: 33-44. - PubMed
-
- Cope T, Pinter MJ (1995) The size principle: still working after all these years? News Physiol Sci 10: 280-286.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
