Interactions between ephrin-B and metabotropic glutamate 1 receptors in brain tissue and cultured neurons
- PMID: 15745950
- PMCID: PMC6726088
- DOI: 10.1523/JNEUROSCI.4956-04.2005
Interactions between ephrin-B and metabotropic glutamate 1 receptors in brain tissue and cultured neurons
Abstract
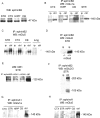
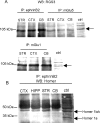
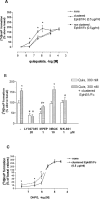
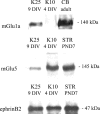
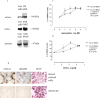
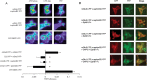
We examined the interaction between ephrins and metabotropic glutamate (mGlu) receptors in the developing brain and cultured neurons. EphrinB2 coimmunoprecipitated with mGlu1a receptors, in all of the brain regions examined, and with mGlu5 receptors in the corpus striatum. In striatal slices, activation of ephrinB2 by a clustered form of its target receptor, EphB1, amplified the mGlu receptor-mediated stimulation of polyphosphoinositide (PI) hydrolysis. This effect was abolished in slices treated with mGlu1 or NMDA receptor antagonists but was not affected by pharmacological blockade of mGlu5 receptors. An interaction among ephrinB2, mGlu1 receptor, and NMDA was supported by the following observations: (1) the NR1 subunit of NMDA receptors coimmunoprecipitated with mGlu1a receptors and ephrinB2 in striatal lysates; (2) clustered EphB1 amplified excitatory amino acid-stimulated PI hydrolysis in cultured granule cells grown under conditions that favored the expression of mGlu1a receptors; and (3) clustered EphB1 amplified the enhancing effect of mGlu receptor agonists on NMDA toxicity in cortical cultures, and its action was sensitive to mGlu1 receptor antagonists. Finally, fluorescence resonance energy transfer and coclustering analysis in human embryonic kidney 293 cells excluded a physical interaction between ephrinB2 and mGlu1a (or mGlu5 receptors). A functional interaction between ephrinB and mGlu1 receptors, which likely involves adaptor or scaffolding proteins, might have an important role in the regulation of developmental plasticity.
Figures
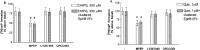
References
-
- Alagarsamy S, Marino MJ, Rouse ST, Gereau IV RW, Heinemann SF, Conn PJ (1999) Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci 2: 234-240. - PubMed
-
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L (2002) Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci 20: 323-329. - PubMed
-
- Aniksztejn L, Sciancalepore M, Ben Ari Y, Cherubini E (1995) Persistent current oscillations produced by activation of metabotropic glutamate receptors in immature rat CA3 hippocampal neurons. J Neurophysiol 73: 1422-1429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
