Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ
- PMID: 15745970
- PMCID: PMC6726089
- DOI: 10.1523/JNEUROSCI.4925-04.2005
Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ
Abstract
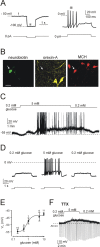
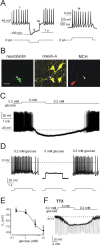
The physiological signaling mechanisms that link normal variations in body energy status to the activity of arousal- and metabolism-regulating brain centers are not well understood. The melanin-concentrating hormone (MCH) and orexin/hypocretin types of neurons of the lateral hypothalamus (LH) exert opposing effects on arousal and metabolism. We examined whether shifts in brain extracellular glucose that correspond to physiological changes in blood glucose can alter the electrical output of neurochemically and biophysically defined LH cells in mouse brain slices. Here, we show that physiologically relevant concentrations of glucose dose-dependently enhance the electrical excitability of MCH neurons by inducing depolarization and increasing membrane resistance. We also demonstrate that the same physiological shifts in glucose have the opposite effects on the electrical activity of orexin neurons. We propose that these direct actions of glucose on the arousal- and metabolism-regulating LH neurons play a key role in the translation of normal variations in body energy resources into appropriate changes in arousal and metabolism.
Figures
Similar articles
-
Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus.J Neurosci. 2008 Sep 10;28(37):9101-10. doi: 10.1523/JNEUROSCI.1766-08.2008. J Neurosci. 2008. PMID: 18784290 Free PMC article.
-
Neurochemical Heterogeneity Among Lateral Hypothalamic Hypocretin/Orexin and Melanin-Concentrating Hormone Neurons Identified Through Single-Cell Gene Expression Analysis.eNeuro. 2017 Sep 22;4(5):ENEURO.0013-17.2017. doi: 10.1523/ENEURO.0013-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 28966976 Free PMC article.
-
Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices.Neuroscience. 2005;130(4):807-11. doi: 10.1016/j.neuroscience.2004.10.032. Neuroscience. 2005. PMID: 15652980
-
Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control.Physiol Behav. 2013 Sep 10;121:117-24. doi: 10.1016/j.physbeh.2013.03.023. Epub 2013 Apr 3. Physiol Behav. 2013. PMID: 23562864 Free PMC article. Review.
-
Local network regulation of orexin neurons in the lateral hypothalamus.Am J Physiol Regul Integr Comp Physiol. 2011 Sep;301(3):R572-80. doi: 10.1152/ajpregu.00674.2010. Epub 2011 Jun 22. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21697524 Review.
Cited by
-
Tuberal hypothalamic neurons secreting the satiety molecule Nesfatin-1 are critically involved in paradoxical (REM) sleep homeostasis.PLoS One. 2012;7(12):e52525. doi: 10.1371/journal.pone.0052525. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300698 Free PMC article.
-
Specification of select hypothalamic circuits and innate behaviors by the embryonic patterning gene dbx1.Neuron. 2015 Apr 22;86(2):403-16. doi: 10.1016/j.neuron.2015.03.022. Epub 2015 Apr 9. Neuron. 2015. PMID: 25864637 Free PMC article.
-
Editorial: The link between nutrition and schizophrenia.Front Psychiatry. 2022 Nov 21;13:1074120. doi: 10.3389/fpsyt.2022.1074120. eCollection 2022. Front Psychiatry. 2022. PMID: 36479557 Free PMC article. No abstract available.
-
Sleep-Wake Cycling and Energy Conservation: Role of Hypocretin and the Lateral Hypothalamus in Dynamic State-Dependent Resource Optimization.Front Neurol. 2018 Oct 5;9:790. doi: 10.3389/fneur.2018.00790. eCollection 2018. Front Neurol. 2018. PMID: 30344503 Free PMC article. Review.
-
Revealing the Antiepileptic Effect of α-Asaronol on Pentylenetetrazole-Induced Seizure Rats Using NMR-Based Metabolomics.ACS Omega. 2022 Feb 9;7(7):6322-6334. doi: 10.1021/acsomega.1c06922. eCollection 2022 Feb 22. ACS Omega. 2022. PMID: 35224394 Free PMC article.
References
-
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE (1992) The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319: 218-245. - PubMed
-
- Burdakov D (2004) Electrical signaling in central orexin/hypocretin circuits: tuning arousal and appetite to fit the environment. The Neuroscientist 10: 286-291. - PubMed
-
- Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK (1999) Orexin A-like immunoreactivity in the rat brain. Neurosci Lett 260: 161-164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
