Coxsackievirus targets proliferating neuronal progenitor cells in the neonatal CNS
- PMID: 15745971
- PMCID: PMC6726081
- DOI: 10.1523/JNEUROSCI.4517-04.2005
Coxsackievirus targets proliferating neuronal progenitor cells in the neonatal CNS
Abstract
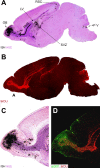
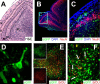
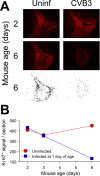
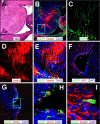
Type B coxsackieviruses (CVB) frequently infect the CNS and, together with other enteroviruses, are the most common cause of viral meningitis in humans. Newborn infants are particularly vulnerable, and CVB also can infect the fetus, leading to mortality, or to neurodevelopmental defects in surviving infants. Using a mouse model of neonatal CVB infection, we previously demonstrated that coxsackievirus B3 (CVB3) could infect neuronal progenitor cells in the subventricular zone (SVZ). Here we extend these findings, and we show that CVB3 targets actively proliferating (bromodeoxyuridine+, Ki67+) cells in the SVZ, including type B and type A stem cells. However, infected cells exiting the SVZ have lost their proliferative capacity, in contrast to their uninfected companions. Despite being proliferation deficient, the infected neuronal precursors could migrate along the rostral migratory stream and radial glia, to reach their final destinations in the olfactory bulb or cerebral cortex. Furthermore, infection did not prevent cell differentiation, as determined by cellular morphology and the expression of maturation markers. These data lead us to propose a model of CVB infection of the developing CNS, which may explain the neurodevelopmental defects that result from fetal infection.
Figures





References
-
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2: 287-293. - PubMed
-
- Daley AJ, Isaacs D, Dwyer DE, Gilbert GL (1998) A cluster of cases of neonatal coxsackievirus B meningitis and myocarditis. J Paediatr Child Health 34: 196-198. - PubMed
-
- Doetsch F (2003) A niche for adult neural stem cells. Curr Opin Genet Dev 13: 543-550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
