Therapeutic potential of a tumor-specific, MHC-unrestricted T-cell receptor expressed on effector cells of the innate and the adaptive immune system through bone marrow transduction and immune reconstitution
- PMID: 15746083
- PMCID: PMC1894994
- DOI: 10.1182/blood-2004-10-3848
Therapeutic potential of a tumor-specific, MHC-unrestricted T-cell receptor expressed on effector cells of the innate and the adaptive immune system through bone marrow transduction and immune reconstitution
Abstract
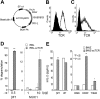
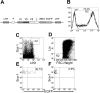
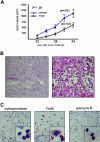
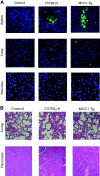
T-cell receptor (TCR) with unique major histocompatibility complex (MHC)-unrestricted antigen-binding properties was isolated from a human T-cell clone specific for the tumor antigen MUC1. This TCR binds its epitope on the MUC1 protein without the requirement of processing and presentation. A single-chain Valpha/Vbeta/Cbeta (scTCR) was fused to a CD3 zeta (zeta) chain to allow expression on the surface of cells of the innate (granulocytes, macrophages, natural killer [NK] cells) as well as the adaptive (T and B cells) immune system. To test the ability of the cells of the innate immune system to reject a tumor when provided with a tumor antigen-specific TCR, we reconstituted severe combined immunodeficiency (SCID) mice with bone marrow cells transduced with a retroviral vector encoding this receptor and challenged them with a MUC1-positive human tumor. These mice controlled the growth of the tumor significantly better than the control mice. We performed a similar experiment in immunocompetent mice transgenic for human MUC1. Expression of the TCR on large percentages of cells did not result in infiltration or destruction of tissues expressing MUC1. Reconstituted mice controlled the outgrowth of a MUC1-transfected but not the parental control tumor. scTCR expression appears lifelong, suggesting a successful transduction of the self-renewing stem cells.
Figures


Similar articles
-
Lentiviral vectors encoding human MUC1-specific, MHC-unrestricted single-chain TCR and a fusion suicide gene: potential for universal and safe cancer immunotherapy.Cancer Immunol Immunother. 2009 Jun;58(6):977-87. doi: 10.1007/s00262-008-0624-0. Epub 2008 Nov 21. Cancer Immunol Immunother. 2009. PMID: 19023569 Free PMC article.
-
Developmental dissociation of T cells from B, NK, and myeloid cells revealed by MHC class II-specific chimeric immune receptors bearing TCR-zeta or FcR-gamma chain signaling domains.Blood. 2002 Oct 15;100(8):3045-8. doi: 10.1182/blood-2002-02-0428. Blood. 2002. PMID: 12351421
-
Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity.J Exp Med. 1991 Jul 1;174(1):139-49. doi: 10.1084/jem.174.1.139. J Exp Med. 1991. PMID: 1711560 Free PMC article.
-
Potential use of T cell receptor genes to modify hematopoietic stem cells for the gene therapy of cancer.Pathol Oncol Res. 1999;5(1):3-15. doi: 10.1053/paor.1999.0003. Pathol Oncol Res. 1999. PMID: 10079371 Review.
-
Tecemotide: an antigen-specific cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3383-93. doi: 10.4161/hv.29836. Hum Vaccin Immunother. 2014. PMID: 25483673 Free PMC article. Review.
Cited by
-
Cloning and expression of human membrane-bound and soluble engineered T cell receptors for immunotherapy.J Biomed Biotechnol. 2006;2006(2):68091. doi: 10.1155/JBB/2006/68091. J Biomed Biotechnol. 2006. PMID: 16883054 Free PMC article.
-
Costimulation with anti-cluster of differentiation 3 and anti-cluster of differentiation 28 reduces the activity of mucin 1-stimulated human mononuclear cells.Oncol Lett. 2016 Jan;11(1):461-465. doi: 10.3892/ol.2015.3840. Epub 2015 Oct 29. Oncol Lett. 2016. PMID: 26870234 Free PMC article.
-
Selection, engineering, and in vivo testing of a human leukocyte antigen-independent T-cell receptor recognizing human mesothelin.PLoS One. 2024 Apr 4;19(4):e0301175. doi: 10.1371/journal.pone.0301175. eCollection 2024. PLoS One. 2024. PMID: 38574067 Free PMC article.
-
Cytotoxic T-lymphocyte immunotherapy for ovarian cancer: a pilot study.J Immunother. 2012 Feb-Mar;35(2):196-204. doi: 10.1097/CJI.0b013e318243f213. J Immunother. 2012. PMID: 22306908 Free PMC article. Clinical Trial.
-
Cancer vaccines: translation from mice to human clinical trials.Curr Opin Immunol. 2018 Apr;51:111-122. doi: 10.1016/j.coi.2018.03.001. Epub 2018 Mar 16. Curr Opin Immunol. 2018. PMID: 29554495 Free PMC article. Review.
References
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344: 783-792. - PubMed
-
- Davis TA, Maloney DG, Czerwinski DK, Liles TM, Levy R. Anti-idiotype antibodies can induce long-term complete remissions in non-Hodgkin's lymphoma without eradicating the malignant clone. Blood. 1998;92: 1184-1190. - PubMed
-
- Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5: 405-411. - PubMed
-
- Saio M, Radoja S, Marino M, Frey AB. Tumor-infiltrating macrophages induce apoptosis in activated CD8(+) T cells by a mechanism requiring cell contact and mediated by both the cell-associated form of TNF and nitric oxide. J Immunol. 2001;167: 5583-5593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

