In vivo recordings from rat geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones
- PMID: 15746166
- PMCID: PMC1464453
- DOI: 10.1113/jphysiol.2005.083741
In vivo recordings from rat geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones
Abstract
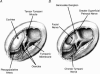
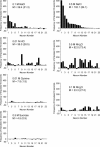
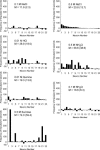
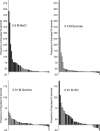
Coding of gustatory information is complex and unique among sensory systems; information is received by multiple receptor populations located throughout the oral cavity and carried to a single central relay by four separate nerves. The geniculate ganglion is the location of the somata of two of these nerves, the greater superficial petrosal (GSP) and the chorda tympani (CT). The GSP innervates taste buds on the palate and the CT innervates taste buds on the anterior tongue. To obtain requisite taste response profiles of GSP neurones, we recorded neurophysiological responses to taste stimuli of individual geniculate ganglion neurones in vivo in the rat and compared them to those from the CT. GSP neurones had a distinct pattern of responding compared to CT neurones. For example, a small subset of GSP neurones had high response frequencies to sucrose stimulation, whereas no CT neurones had high response frequencies to sucrose. In contrast, NaCl elicited high response frequencies in a small subset of CT neurones and elicited moderate response frequencies in a relatively large proportion of GSP neurones. The robust whole-nerve response to sucrose in the GSP may be attributable to relatively few, narrowly tuned neurones, whereas the response to NaCl in the GSP may relate to proportionately more, widely tuned neurones. These results demonstrate the diversity in the initial stages of sensory coding for two separate gustatory nerves involved in the ingestion or rejection of taste solutions, and may have implications for central coding of gustatory quality and concentration as well as coding of information used in controlling energy, fluid and electrolyte homeostasis.
Figures



References
-
- Beidler LM. Properties of chemoreceptors of tongue of rat. J Neurophysiol. 1953;16:595–607. - PubMed
-
- Boudreau JC, Hoang NK, Oravec J, Do LT. Rat neurophysiological taste responses to salt solutions. Chem Senses. 1983;8:131–150.
-
- Bradley RM. Sensory receptors of the larynx. Am J Med. 2000;108:47S–50S. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

