The vagus regulates histamine mobilization from rat stomach ECL cells by controlling their sensitivity to gastrin
- PMID: 15746169
- PMCID: PMC1464455
- DOI: 10.1113/jphysiol.2005.082677
The vagus regulates histamine mobilization from rat stomach ECL cells by controlling their sensitivity to gastrin
Abstract
The ECL cells in the oxyntic mucosa secrete histamine in response to gastrin, stimulating parietal cells to produce acid. Do they also operate under nervous control? The present study examines histamine mobilization from rat stomach ECL cells in situ in response to acute vagal excitation and to food or gastrin following vagal or sympathetic denervation. Applying the technique of microdialysis, we monitored the release of histamine by radioimmunoassay. Microdialysis probes were placed in the submucosa on either side of the stomach, 3 days before experiments. The rats were awake during microdialysis except when subjected to electrical vagal stimulation. One-sided electrical vagal stimulation raised serum gastrin and mobilized gastric histamine. However, gastrin receptor blockade prevented the histamine mobilization, indicating that circulating gastrin accounts for the response. Vagal excitation by hypoglycaemia (insulin) or pylorus ligation did not mobilize either gastrin or histamine. The histamine response to food was almost abolished by gastrin receptor blockade, and it was halved on the denervated side after unilateral subdiaphragmatic vagotomy. While the histamine response to a near-maximally effective dose of gastrin was unaffected by vagotomy, the response to low gastrin doses was reduced significantly. Abdominal ganglionic sympathectomy failed to affect the histamine response to either food or gastrin. In conclusion, gastrin is responsible for most of the food-evoked mobilization of ECL-cell histamine. The histamine response to electrical vagal stimulation reflects the effect of circulating gastrin rather than a direct action of the vagus on the ECL cells. Vagal denervation was accompanied by an impaired histamine response to food intake, probably reflecting the right-ward shift of the serum gastrin concentration-histamine response curve. The results suggest that the vagus controls the sensitivity of the ECL cells to gastrin.
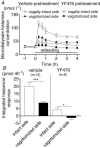
Figures




Similar articles
-
Submucosal microinfusion of endothelin and adrenaline mobilizes ECL-cell histamine in rat stomach, and causes mucosal damage: a microdialysis study.Br J Pharmacol. 2003 Oct;140(4):707-17. doi: 10.1038/sj.bjp.0705473. Epub 2003 Sep 22. Br J Pharmacol. 2003. PMID: 14504142 Free PMC article.
-
Gastrin release: Antrum microdialysis reveals a complex neural control.Regul Pept. 2010 Apr 9;161(1-3):22-32. doi: 10.1016/j.regpep.2010.01.004. Epub 2010 Jan 18. Regul Pept. 2010. PMID: 20085791
-
Anaesthetic agents inhibit gastrin-stimulated but not basal histamine release from rat stomach ECL cells.Br J Pharmacol. 2000 Jun;130(4):725-30. doi: 10.1038/sj.bjp.0703347. Br J Pharmacol. 2000. PMID: 10864877 Free PMC article.
-
Physiological significance of ECL-cell histamine.Yale J Biol Med. 1998 May-Aug;71(3-4):183-93. Yale J Biol Med. 1998. PMID: 10461351 Free PMC article. Review.
-
The biology and physiology of the ECL cell.Yale J Biol Med. 1994 May-Aug;67(3-4):123-34. Yale J Biol Med. 1994. PMID: 7502521 Free PMC article. Review.
Cited by
-
Lack of cholinergic innervation in gastric mucosa does not affect gastrin secretion or basal acid output in neurturin receptor GFRα2 deficient mice.J Physiol. 2013 Apr 15;591(8):2175-88. doi: 10.1113/jphysiol.2012.246801. Epub 2013 Jan 21. J Physiol. 2013. PMID: 23339174 Free PMC article.
-
Gastric secretions affected by esophageal distention in the rat.J Gastroenterol. 2009;44(2):132-8. doi: 10.1007/s00535-008-2288-0. Epub 2009 Feb 13. J Gastroenterol. 2009. PMID: 19214675
-
Tachyphylaxis of the ECL-cell response to PACAP: receptor desensitization and/or depletion of secretory products.Br J Pharmacol. 2007 Sep;152(2):240-8. doi: 10.1038/sj.bjp.0707385. Epub 2007 Jul 30. Br J Pharmacol. 2007. PMID: 17660849 Free PMC article.
-
The identification of neuronal control pathways supplying effector tissues in the stomach.Cell Tissue Res. 2020 Dec;382(3):433-445. doi: 10.1007/s00441-020-03294-7. Epub 2020 Nov 6. Cell Tissue Res. 2020. PMID: 33156383 Free PMC article. Review.
-
Evaluation of Gastric pH and Gastrin Concentrations in Horses Subjected to General Inhalation Anesthesia in Dorsal Recumbency.Animals (Basel). 2024 Apr 15;14(8):1183. doi: 10.3390/ani14081183. Animals (Basel). 2024. PMID: 38672331 Free PMC article.
References
-
- Alino SF, Garcia D, Uvnas-Moberg K. On the interaction between intragastric pH and electrical vagal stimulation in causing gastric acid secretion and intraluminal release of gastrin and somatostatin in anesthetized rats. Acta Physiol Scand. 1983;117:491–495. - PubMed
-
- Axelson J, Ekelund M, Håkanson R, Sundler F. Gastrin and the vagus interact in the trophic control of the rat oxyntic mucosa. Regul Pept. 1988;22:237–243. 10.1016/0167-0115(88)90036-5. - DOI - PubMed
-
- Brodie DA, Knapp PG. The mechanism of the inhibition of gastric secretion produced by esophageal ligation in the pylorus-ligated rat. Gastroenterology. 1966;50:787–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

