Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin
- PMID: 15746172
- PMCID: PMC1464525
- DOI: 10.1113/jphysiol.2004.081422
Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin
Abstract
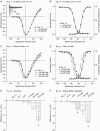
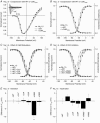
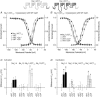
Calmodulin (CaM) has been shown to modulate different ion channels, including voltage-gated sodium channels (NaChs). Using the yeast two-hybrid assay, we found an interaction between CaM and the C-terminal domains of adult skeletal (NaV1.4) and cardiac (NaV1.5) muscle NaChs. Effects of CaM were studied using sodium channels transiently expressed in CHO cells. Wild type CaM (CaM(WT)) caused a hyperpolarizing shift in the voltage dependence of activation and inactivation for NaV1.4 and activation for NaV1.5. Intracellular application of CaM caused hyperpolarizing shifts equivalent to those seen with CaM(WT) coexpression with NaV1.4. Elevated Ca2+ and CaM-binding peptides caused depolarizing shifts in the inactivation curves seen with CaM(WT) coexpression with NaV1.4. KN93, a CaM-kinase II inhibitor, had no effect on NaV1.4, suggesting that CaM acts directly on NaV1.4 and not through activation of CaM-kinase II. Coexpression of hemi-mutant CaMs showed that an intact N-terminal lobe of CaM is required for effects of CaM upon NaV1.4. Mutations in the sodium channel IQ domain disrupted the effects of CaM on NaV1.4: the I1727E mutation completely blocked all calmodulin effects, while the L1736R mutation disrupted the effects of Ca2+-calmodulin on inactivation. Chimeric channels of NaV1.4 and NaV1.5 also indicated that the C-terminal domain is largely responsible for CaM effects on inactivation. CaM had little effect on NaV1.4 expressed in HEK cells, possibly due to large differences in the endogenous expression of beta-subunits between CHO and HEK cells. These results in heterologous cells suggest that Ca2+ released during muscle contraction rapidly modulates NaCh availability via CaM.
Figures






References
-
- Carlier E, Dargent B, De Waard M, Couraud F. Na+ channel regulation by calmodulin kinase II in rat cerebellar granule cells. Biochem Biophys Res Commun. 2000;274:394–399. - PubMed
-
- Chou JJ, Li S, Klee CB, Bax A. Solution structure of Ca2+-calmodulin reveals flexible hand-like properties of its domains. Nature Struc Biol. 2001;8:990–996. - PubMed
-
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

