CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes
- PMID: 15746174
- PMCID: PMC1464468
- DOI: 10.1113/jphysiol.2004.079046
CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes
Abstract
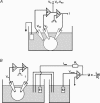
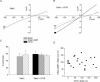
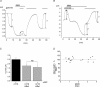
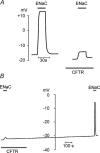
The cystic fibrosis transmembrane conductance regulator (CFTR) plays a crucial role in regulating fluid secretion by the airways, intestines, sweat glands and other epithelial tissues. It is well established that the CFTR is a cAMP-activated, nucleotide-dependent anion channel, but additional functions are often attributed to it, including regulation of the epithelial sodium channel (ENaC). The absence of CFTR-dependent ENaC inhibition and the resulting sodium hyperabsorption were postulated to be a major electrolyte transport abnormality in cystic fibrosis (CF)-affected epithelia. Several ex vivo studies, including those that used the Xenopus oocyte expression system, have reported ENaC inhibition by activated CFTR, but contradictory results have also been obtained. Because CFTR-ENaC interactions have important implications in the pathogenesis of CF, the present investigation was undertaken by our three independent laboratories to resolve whether CFTR regulates ENaC in oocytes and to clarify potential sources of previously reported dissimilar observations. Using different experimental protocols and a wide range of channel expression levels, we found no evidence that activated CFTR regulates ENaC when oocyte membrane potential was carefully clamped. We determined that an apparent CFTR-dependent ENaC inhibition could be observed when resistance in series with the oocyte membrane was not low enough or the feedback voltage gain was not high enough. We suggest that the inhibitory effect of CFTR on ENaC reported in some earlier oocyte studies could be attributed to problems arising from high levels of channel expression and suboptimal recording conditions, that is, large series resistance and/or insufficient feedback voltage gain.
Figures
References
-
- Akabas MH. Cystic fibrosis transmembrane conductance regulator. Structure and function of an epithelial chloride channel. J Biol Chem. 2000;275:3729–3732. 10.1074/jbc.275.6.3729. - DOI - PubMed
-
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. - PubMed
-
- Armstrong CM, Gilly WF. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol. 1992;207:100–122. - PubMed
-
- Axon Instruments. The Axon Guide. Foster City CA: Axon Instruments Inc; 1993. pp. 1–282.
-
- Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991;352:70–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

