Spatially resolved characterization of biogenic manganese oxide production within a bacterial biofilm
- PMID: 15746332
- PMCID: PMC1065152
- DOI: 10.1128/AEM.71.3.1300-1310.2005
Spatially resolved characterization of biogenic manganese oxide production within a bacterial biofilm
Abstract
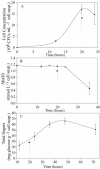
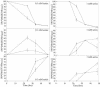
Pseudomonas putida strain MnB1, a biofilm-forming bacterial culture, was used as a model for the study of bacterial Mn oxidation in freshwater and soil environments. The oxidation of aqueous Mn+2 [Mn+2(aq)] by P. putida was characterized by spatially and temporally resolving the oxidation state of Mn in the presence of a bacterial biofilm, using scanning transmission X-ray microscopy (STXM) combined with near-edge X-ray absorption fine structure (NEXAFS) spectroscopy at the Mn L2,3 absorption edges. Subsamples were collected from growth flasks containing 0.1 and 1 mM total Mn at 16, 24, 36, and 48 h after inoculation. Immediately after collection, the unprocessed hydrated subsamples were imaged at a 40-nm resolution. Manganese NEXAFS spectra were extracted from X-ray energy sequences of STXM images (stacks) and fit with linear combinations of well-characterized reference spectra to obtain quantitative relative abundances of Mn(II), Mn(III), and Mn(IV). Careful consideration was given to uncertainty in the normalization of the reference spectra, choice of reference compounds, and chemical changes due to radiation damage. The STXM results confirm that Mn+2(aq) was removed from solution by P. putida and was concentrated as Mn(III) and Mn(IV) immediately adjacent to the bacterial cells. The Mn precipitates were completely enveloped by bacterial biofilm material. The distribution of Mn oxidation states was spatially heterogeneous within and between the clusters of bacterial cells. Scanning transmission X-ray microscopy is a promising tool for advancing the study of hydrated interfaces between minerals and bacteria, particularly in cases where the structure of bacterial biofilms needs to be maintained.
Figures





References
-
- Abbate, M., F. M. F. deGroot, J. C. Fuggle, A. Fujimori, Y. Tokura, Y. Fujishima, O. Strebel, M. Domke, G. Kaindl, J. van Elp, B. T. Thole, G. A. Sawatsky, M. Sacchi, and N. Tsuda. 1991. Soft-x-ray absorption studies of the location of extra charges induced by substitution in controlled-valence materials. Phys. Rev. B Condens. Matter 44:419-5422. - PubMed
-
- Banerjee, D., and H. W. Nesbitt. 1999. Oxidation of aqueous Cr(III) at birnessite surfaces: constraints on reaction mechanism. Geochim. Cosmochim. Acta 63:1671-1687.
-
- Banfield, J. F., and R. J. Hamers. 1997. Processes at minerals and surfaces with relevance to microorganisms and prebiotic synthesis, p. 81-122. In J. F. Banfield and K. H. Nealson (ed.), Geomicrobiology: interactions between microbes and minerals. Reviews in Mineralogy, vol. 35. Mineralogical Society of America, Washington, D.C.
-
- Bargar, J. R., B. M. Tebo, U. Bergmann, S. M. Webb, P. Glatzel, V. Q. Chiu, and M. Villalobos. 2005. Biotic and abiotic products of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Am. Mineral. 90:143-154.

