Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water
- PMID: 15746342
- PMCID: PMC1065171
- DOI: 10.1128/AEM.71.3.1394-1404.2005
Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water
Abstract
A repeated cross-sectional study was conducted to determine the patterns of antimicrobial resistance in 1,286 Escherichia coli strains isolated from human septage, wildlife, domestic animals, farm environments, and surface water in the Red Cedar watershed in Michigan. Isolation and identification of E. coli were done by using enrichment media, selective media, and biochemical tests. Antimicrobial susceptibility testing by the disk diffusion method was conducted for neomycin, gentamicin, streptomycin, chloramphenicol, ofloxacin, trimethoprim-sulfamethoxazole, tetracycline, ampicillin, nalidixic acid, nitrofurantoin, cephalothin, and sulfisoxazole. Resistance to at least one antimicrobial agent was demonstrated in isolates from livestock, companion animals, human septage, wildlife, and surface water. In general, E. coli isolates from domestic species showed resistance to the largest number of antimicrobial agents compared to isolates from human septage, wildlife, and surface water. The agents to which resistance was demonstrated most frequently were tetracycline, cephalothin, sulfisoxazole, and streptomycin. There were similarities in the patterns of resistance in fecal samples and farm environment samples by animal, and the levels of cephalothin-resistant isolates were higher in farm environment samples than in fecal samples. Multidrug resistance was seen in a variety of sources, and the highest levels of multidrug-resistant E. coli were observed for swine fecal samples. The fact that water sample isolates were resistant only to cephalothin may suggest that the resistance patterns for farm environment samples may be more representative of the risk of contamination of surface waters with antimicrobial agent-resistant bacteria.
Figures

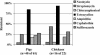

References
-
- Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7:410-413. - PubMed
-
- Barton, M. D. 1998. Does the use of antibiotics in animals affect human health? Aust. Vet. J. 76:177-180. - PubMed
-
- Beharav, A., and E. Nevo. 2003. Predictive validity of discriminant analysis for genetic data. Genetica 119:259-267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

