Posttranslational modification of 6-phosphofructo-1-kinase in Aspergillus niger
- PMID: 15746345
- PMCID: PMC1065176
- DOI: 10.1128/AEM.71.3.1425-1432.2005
Posttranslational modification of 6-phosphofructo-1-kinase in Aspergillus niger
Abstract
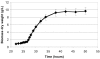
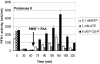
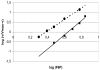
Two different enzymes exhibiting 6-phosphofructo-1-kinase (PFK1) activity were isolated from the mycelium of Aspergillus niger: the native enzyme with a molecular mass of 85 kDa, which corresponded to the calculated molecular mass of the deduced amino acid sequence of the A. niger pfkA gene, and a shorter protein of approximately 49 kDa. A fragment of identical size also was obtained in vitro by the proteolytic digestion of the partially purified native PFK1 with proteinase K. When PFK1 activity was measured during the proteolytic degradation of the native protein, it was found to be lost after 1 h of incubation, but it was reestablished after induction of phosphorylation by adding the catalytic subunit of cyclic AMP-dependent protein kinase to the system. By determining kinetic parameters, different ratios of activities measured at ATP concentrations of 0.1 and 1 mM were detected with fragmented PFK1, as with the native enzyme. Fructose-2,6-biphosphate significantly increased the Vmax of the fragmented protein, while it had virtually no effect on the native protein. The native enzyme could be purified only from the early stages of growth on a minimal medium, while the 49-kDa fragment appeared later and was activated at the time of a sudden change in the growth rate. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of sequential purifications of PFK1 enzymes by affinity chromatography during the early stages of the fungal development suggested spontaneous posttranslational modification of the native PFK1 in A. niger cells, while from the kinetic parameters determined for both isolated forms it could be concluded that the fragmented enzyme might be more efficient under physiological conditions.
Figures




Similar articles
-
Posttranslational modification of 6-phosphofructo-1-kinase as an important feature of cancer metabolism.PLoS One. 2011 May 4;6(5):e19645. doi: 10.1371/journal.pone.0019645. PLoS One. 2011. PMID: 21573193 Free PMC article.
-
citrate inhibition-resistant form of 6-phosphofructo-1-kinase from Aspergillus niger.Appl Environ Microbiol. 2006 Jul;72(7):4515-21. doi: 10.1128/AEM.00539-06. Appl Environ Microbiol. 2006. PMID: 16820438 Free PMC article.
-
Highly active, citrate inhibition resistant form of Aspergillus niger 6-phosphofructo-1-kinase encoded by a modified pfkA gene.J Biotechnol. 2009 Oct 12;144(1):51-7. doi: 10.1016/j.jbiotec.2009.04.004. Epub 2009 Apr 18. J Biotechnol. 2009. PMID: 19379783
-
Evidence for the activation of 6-phosphofructo-1-kinase by cAMP-dependent protein kinase in Aspergillus niger.FEMS Microbiol Lett. 1994 May 15;118(3):327-33. doi: 10.1016/0378-1097(94)90524-x. FEMS Microbiol Lett. 1994. PMID: 8020755
-
Rat liver 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase: a review of relationships between the two activities of the enzyme.J Cell Biochem. 1984;26(1):1-17. doi: 10.1002/jcb.240260102. J Cell Biochem. 1984. PMID: 6096384 Review.
Cited by
-
Analysis of the regulatory mechanism of deoxynivalenol production using omics.AMB Express. 2018 Oct 3;8(1):161. doi: 10.1186/s13568-018-0688-y. AMB Express. 2018. PMID: 30284112 Free PMC article.
-
Blocking hexose entry into glycolysis activates alternative metabolic conversion of these sugars and upregulates pentose metabolism in Aspergillus nidulans.BMC Genomics. 2018 Mar 22;19(1):214. doi: 10.1186/s12864-018-4609-x. BMC Genomics. 2018. PMID: 29566661 Free PMC article.
-
Designer Glycolysomes: Colocalisation of Glycolytic Enzymes on a Cellulosome-Based Synthetic Protein Scaffold.Microb Biotechnol. 2025 Apr;18(4):e70134. doi: 10.1111/1751-7915.70134. Microb Biotechnol. 2025. PMID: 40162578 Free PMC article.
-
Posttranslational modification of 6-phosphofructo-1-kinase as an important feature of cancer metabolism.PLoS One. 2011 May 4;6(5):e19645. doi: 10.1371/journal.pone.0019645. PLoS One. 2011. PMID: 21573193 Free PMC article.
-
citrate inhibition-resistant form of 6-phosphofructo-1-kinase from Aspergillus niger.Appl Environ Microbiol. 2006 Jul;72(7):4515-21. doi: 10.1128/AEM.00539-06. Appl Environ Microbiol. 2006. PMID: 16820438 Free PMC article.
References
-
- Arts, E., C. P. Kubicek, and M. Röhr. 1987. Regulation of phosphofructokinase from Aspergillus niger: effect of fructose-2,6-biphosphate on the action of citrate, ammonium ions and AMP. Microbiology 133:1195-1199.
-
- Atkinson, T., P. M. Hammond, R. D. Hartwell, P. Hughes, M. D. Scawen, R. F. Sherwood, D. A. P. Small, C. J. Bruton, M. J. Harvey, and C. R. Lowe. 1981. Triazine dye affinity chromatography. Biochem. Soc. Trans. 9:290-293. - PubMed
-
- Emerk, K., and C. Frieden. 1974. The effect of trypsin treatment on rabbit muscle phosphofructokinase. Arch. Biochem. Biophys. 164:233-240. - PubMed
-
- Fernandez, M., J. Cao, and J. A. Villamarin. 1998. In vivo phosphorylation of phosphofructokinase from the bivalve mollusc Mytilus galloprovincialis. Arch. Biochem. Biophys. 353:251-256. - PubMed
-
- Freyer, R., H. Zietz, H. J. Zietz, and E. Hofmann. 1978. Modification of yeast phosphofructokinase by trypsin and subtilisin in the presence of effectors. Acta Biol. Med. Ger. 37:993-999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

