Functional characterization of two cytochrome P450 monooxygenase genes, P450-1 and P450-4, of the gibberellic acid gene cluster in Fusarium proliferatum (Gibberella fujikuroi MP-D)
- PMID: 15746349
- PMCID: PMC1065163
- DOI: 10.1128/AEM.71.3.1462-1472.2005
Functional characterization of two cytochrome P450 monooxygenase genes, P450-1 and P450-4, of the gibberellic acid gene cluster in Fusarium proliferatum (Gibberella fujikuroi MP-D)
Abstract
Gibberella fujikuroi is a species complex with at least nine different biological species, termed mating populations (MPs) A to I (MP-A to MP-I), known to produce many different secondary metabolites. So far, gibberellin (GA) production is restricted to Fusarium fujikuroi (G. fujikuroi MP-C), although at least five other MPs contain all biosynthetic genes. Here, we analyze the GA gene cluster and GA pathway in the closest related species, Fusarium proliferatum (MP-D), and demonstrate that the GA genes share a high degree of sequence homology with the corresponding genes of MP-C. The GA production capacity was restored after integration of the entire GA gene cluster from MP-C, indicating the existence of an active regulation system in F. proliferatum. The results further indicate that one reason for the loss of GA production is the accumulation of several mutations in the coding and 5' noncoding regions of the ent-kaurene oxidase gene, P450-4.
Figures



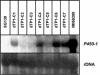
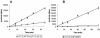


References
-
- Backes, W. L., and R. W. Kelley. 2003. Organization of multiple cytochrome P450s with NADPH-cytochrome P450 reductase in membranes. Pharmacol. Ther. 98:221-233. - PubMed
-
- Barendse, G. W. M., P. H. van de Werken, and N. Takahashi. 1989. High-performance liquid chromatography of gibberellins. J. Chromatogr. 198:449-455.
-
- Bhatnagar, D., K. C. Ehrlich., and T. E. Cleveland. 2003. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 61:83-93. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

