Intravitreal human immune globulin in a rabbit model of Staphylococcus aureus toxin-mediated endophthalmitis: a potential adjunct in the treatment of endophthalmitis
- PMID: 15747765
- PMCID: PMC1280107
Intravitreal human immune globulin in a rabbit model of Staphylococcus aureus toxin-mediated endophthalmitis: a potential adjunct in the treatment of endophthalmitis
Abstract
Objectives: To test the feasibility of human immune globulin (IG, Gamimune N, 10%) as a new treatment for endophthalmitis, the ocular tolerance, distribution, and ability of intravitreal IG to attenuate the toxic effects of Staphylococcus aureus culture supernatant were evaluated in a rabbit model.
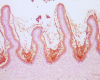
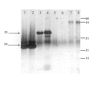
Methods: Effects of intravitreally injected IG were assessed histologically and with Western blot analysis performed 1 to 5 days after injection. IG reactivity to products of S. aureus strain RN4220 was tested by Western blotting, using known toxins (beta hemolysin and toxic shock syndrome toxin-1) and a concentrated culture supernatant containing S. aureus exotoxins (pooled toxin, PT). Endophthalmitis was induced by intravitreal PT injection. For treatment, IG and PT were mixed and injected simultaneously, or IG was injected immediately after, or 6 hours after, PT injection. PT toxicity was graded clinically and histologically over 9 days.
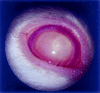
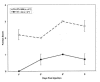
Results: IG persisted intravitreally at least 5 days, inducing no clinical inflammation and minimal mononuclear cell infiltration. In the endophthalmitis model, toxicity from PT was significantly reduced when IG was mixed with PT and injected simultaneously, or when IG was delivered immediately after PT. Only minimal clinically detectable reductions were observed when IG delivery was delayed 6 hours.
Conclusions: Intravitreal IG is well tolerated in the rabbit eye and attenuates the toxicity of culture supernatant containing S. aureus exotoxins. Because toxin elaboration likely occurs gradually in true infection, reduced effects observed with delayed treatment in this toxin-injected model do not preclude clinical application. IG may represent a novel adjunct in endophthalmitis treatment.
Figures











References
-
- Endophthalmitis Vitrectomy Study Group. . Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. - PubMed
-
- Endophthalmitis Vitrectomy Study Group. . Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:830–846. - PubMed
-
- Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic factors isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:1–17. - PubMed
-
- Kresloff MS, Castellarin AA, Zarbin M. Endophthalmitis. Surv Ophthalmol. 1998;43:193–224. - PubMed
-
- Waheed S, Ritterband DC, Greenfield DS, et al. New patterns of infecting organisms in late bleb-related endophthalmitis: a ten year review. Eye. 1998;12:910–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
