Heterologous expression in Tritrichomonas foetus of functional Trichomonas vaginalis AP65 adhesin
- PMID: 15748280
- PMCID: PMC1079839
- DOI: 10.1186/1471-2199-6-5
Heterologous expression in Tritrichomonas foetus of functional Trichomonas vaginalis AP65 adhesin
Abstract
Background: Trichomonosis, caused by Trichomonas vaginalis, is the number one, nonviral sexually transmitted infection that has adverse consequences for the health of women and children. The interaction of T. vaginalis with vaginal epithelial cells (VECs), a step preparatory to infection, is mediated in part by the prominent surface protein AP65. The bovine trichomonad, Tritrichomonas foetus, adheres poorly to human VECs. Thus, we established a transfection system for heterologous expression of the T. vaginalis AP65 in T. foetus, as an alternative approach to confirm adhesin function for this virulence factor.
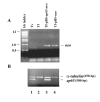
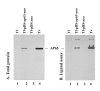
Results: In this study, we show stable transfection and expression of the T. vaginalis ap65 gene in T. foetus from an episomal pBS-ap65-neo plasmid. Expression of the gene and protein was confirmed by RT-PCR and immunoblots, respectively. AP65 in transformed T. foetus bound to host cells. Specific mAbs revealed episomally-expressed AP65 targeted to the parasite surface and hydrogenosome organelles. Importantly, surface-expression of AP65 in T. foetus paralleled increased levels of adherence of transfected bovine trichomonads to human VECs.
Conclusion: The T. vaginalis AP65 adhesin was stably expressed in T. foetus, and the data obtained using this heterologous system strongly supports the role of AP65 as a prominent adhesin for T. vaginalis. In addition, the heterologous expression in T. foetus of a T. vaginalis gene offers an important, new approach for confirming and characterizing virulence factors.
Figures





Similar articles
-
Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis.Mol Microbiol. 2004 Aug;53(4):1099-108. doi: 10.1111/j.1365-2958.2004.04192.x. Mol Microbiol. 2004. PMID: 15306014 Free PMC article.
-
Antisense RNA decreases AP33 gene expression and cytoadherence by T. vaginalis.BMC Microbiol. 2007 Jul 3;7:64. doi: 10.1186/1471-2180-7-64. BMC Microbiol. 2007. PMID: 17608941 Free PMC article.
-
Molecular characterization of a third malic enzyme-like AP65 adhesin gene of Trichomonas vaginalis.Microb Pathog. 1996 Jun;20(6):335-49. doi: 10.1006/mpat.1996.0032. Microb Pathog. 1996. PMID: 8831829
-
Trichomonads under Microscopy.Microsc Microanal. 2004 Oct;10(5):528-50. doi: 10.1017/S1431927604040905. Microsc Microanal. 2004. PMID: 15525428 Review.
-
Use of an animal model of trichomoniasis as a basis for understanding this disease in women.Clin Infect Dis. 1995 Oct;21 Suppl 2:S158-61. doi: 10.1093/clinids/21.supplement_2.s158. Clin Infect Dis. 1995. PMID: 8845444 Review.
Cited by
-
Regulation of nuclear translocation of the Myb1 transcription factor by TvCyclophilin 1 in the protozoan parasite Trichomonas vaginalis.J Biol Chem. 2014 Jul 4;289(27):19120-36. doi: 10.1074/jbc.M114.549410. Epub 2014 May 15. J Biol Chem. 2014. PMID: 24831011 Free PMC article.
-
Anti-Retroviral Lectins Have Modest Effects on Adherence of Trichomonas vaginalis to Epithelial Cells In Vitro and on Recovery of Tritrichomonas foetus in a Mouse Vaginal Model.PLoS One. 2015 Aug 7;10(8):e0135340. doi: 10.1371/journal.pone.0135340. eCollection 2015. PLoS One. 2015. PMID: 26252012 Free PMC article.
-
Immunogenic and plasminogen-binding surface-associated alpha-enolase of Trichomonas vaginalis.Infect Immun. 2008 Feb;76(2):523-31. doi: 10.1128/IAI.01352-07. Epub 2007 Dec 10. Infect Immun. 2008. PMID: 18070902 Free PMC article.
-
The Pathogenesis of Human Cervical Epithelium Cells Induced by Interacting with Trichomonas vaginalis.PLoS One. 2015 Apr 22;10(4):e0124087. doi: 10.1371/journal.pone.0124087. eCollection 2015. PLoS One. 2015. PMID: 25901354 Free PMC article.
-
Characterization of the Trichomonas vaginalis surface-associated AP65 and binding domain interacting with trichomonads and host cells.BMC Microbiol. 2007 Dec 25;7:116. doi: 10.1186/1471-2180-7-116. BMC Microbiol. 2007. PMID: 18158858 Free PMC article.
References
-
- Sorvillo F, Kovacs A, Kerndt P, Stek A, Muderspach L, Sanchez-Keeland L. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495–500. - PubMed
-
- El-Shazly AM, El-Naggar HM, Soliman M, El-Negeri M, El-Nemr HE, Handousa AE, Morsy TA. A study on Trichomonas vaginalis and female infertility. J Egypt Soc Parasitol. 2001;31:545–553. - PubMed
-
- Sherman KJ, Chow WH, Daling JR, Weiss NS. Sexually transmitted diseases and the risk of tubal pregnancy. J Reprod Med. 1988;33:30–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

