Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana
- PMID: 15749757
- PMCID: PMC1088001
- DOI: 10.1105/tpc.104.029637
Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana
Abstract
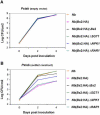
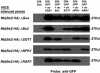
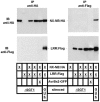
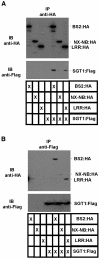
Pepper plants (Capsicum annuum) containing the Bs2 resistance gene are resistant to strains of Xanthomonas campestris pv vesicatoria (Xcv) expressing the bacterial effector protein AvrBs2. AvrBs2 is delivered directly to the plant cell via the type III protein secretion system (TTSS) of Xcv. Upon recognition of AvrBs2 by plants expressing the Bs2 gene, a signal transduction cascade is activated leading to a bacterial disease resistance response. Here, we describe a novel pathosystem that consists of epitope-tagged Bs2-expressing transgenic Nicotiana benthamiana plants and engineered strains of Pseudomonas syringae pv tabaci that deliver the effector domain of the Xcv AvrBs2 protein via the TTSS of P. syringae. This pathosystem has allowed us to exploit N. benthamiana as a model host plant to use Agrobacterium tumefaciens-mediated transient protein expression in conjunction with virus-induced gene silencing to validate genes and to identify protein interactions required for the expression of plant host resistance. In this study, we demonstrate that two genes, NbSGT1 and NbNPK1, are required for the Bs2/AvrBs2-mediated resistance responses but that NbRAR1 is not. Protein localization studies in these plants indicate that full-length Bs2 is primarily localized in the plant cytoplasm. Three protein domains of Bs2 have been identified: the N terminus, a central nucleotide binding site, and a C-terminal Leu-rich repeat (LRR). Co-immunoprecipitation studies demonstrate that separate epitope-tagged Bs2 domain constructs interact in trans specifically in the plant cell. Co-immunoprecipitation studies also demonstrate that an NbSGT1-dependent intramolecular interaction is required for Bs2 function. Additionally, Bs2 has been shown to associate with SGT1 via the LRR domain of Bs2. These data suggest a role for SGT1 in the proper folding of Bs2 or the formation of a Bs2-SGT1-containing protein complex that is required for the expression of bacterial disease resistance.
Figures




Similar articles
-
Inducible expression of Bs2 R gene from Capsicum chacoense in sweet orange (Citrus sinensis L. Osbeck) confers enhanced resistance to citrus canker disease.Plant Mol Biol. 2017 Apr;93(6):607-621. doi: 10.1007/s11103-017-0586-8. Epub 2017 Feb 2. Plant Mol Biol. 2017. PMID: 28155188
-
The conserved Xanthomonas campestris pv. vesicatoria effector protein XopX is a virulence factor and suppresses host defense in Nicotiana benthamiana.Plant J. 2005 Mar;41(6):801-14. doi: 10.1111/j.1365-313X.2005.02338.x. Plant J. 2005. PMID: 15743446
-
Molecular signals required for type III secretion and translocation of the Xanthomonas campestris AvrBs2 protein to pepper plants.Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):13324-9. doi: 10.1073/pnas.230450797. Proc Natl Acad Sci U S A. 2000. PMID: 11078519 Free PMC article.
-
Role of SGT1 in the regulation of plant R gene signalling.Microbes Infect. 2003 Sep;5(11):969-76. doi: 10.1016/s1286-4579(03)00183-7. Microbes Infect. 2003. PMID: 12941389 Review.
-
Controlling the interplay between Agrobacterium tumefaciens and plants during the transient expression of proteins.Bioengineered. 2015;6(4):242-4. doi: 10.1080/21655979.2015.1052920. Epub 2015 May 22. Bioengineered. 2015. PMID: 25997443 Free PMC article. Review.
Cited by
-
Differential modulation of plant immune responses by diverse members of the Pseudomonas savastanoi pv. savastanoi HopAF type III effector family.Mol Plant Pathol. 2017 Jun;18(5):625-634. doi: 10.1111/mpp.12420. Epub 2016 Jul 26. Mol Plant Pathol. 2017. PMID: 27116193 Free PMC article.
-
Recognition of the Hyaloperonospora parasitica effector ATR13 triggers resistance against oomycete, bacterial, and viral pathogens.Proc Natl Acad Sci U S A. 2008 Jan 22;105(3):1091-6. doi: 10.1073/pnas.0711215105. Epub 2008 Jan 15. Proc Natl Acad Sci U S A. 2008. PMID: 18198274 Free PMC article.
-
RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex.Plant Cell. 2008 Aug;20(8):2265-79. doi: 10.1105/tpc.107.054395. Epub 2008 Aug 22. Plant Cell. 2008. PMID: 18723578 Free PMC article.
-
Animal NLRs provide structural insights into plant NLR function.Ann Bot. 2017 Mar 1;119(5):827-702. doi: 10.1093/aob/mcw171. Ann Bot. 2017. PMID: 27562749 Free PMC article. Review.
-
The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance.EMBO J. 2014 Sep 1;33(17):1941-59. doi: 10.15252/embj.201487923. Epub 2014 Jul 14. EMBO J. 2014. PMID: 25024433 Free PMC article.
References
-
- Axtell, M.J., and Staskawicz, B.J. (2003). Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112, 369–377. - PubMed
-
- Azevedo, C., Sadanandom, A., Kitagawa, K., Freialdenhoven, A., Shirasu, K., and Schulze-Lefert, P. (2002). The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295, 2073–2076. - PubMed
-
- Bieri, S., Mauch, S., Shen, Q.H., Peart, J., Devoto, A., Casais, C., Ceron, F., Schulze, S., Steinbiss, H.-H., Shirasu, K., and Schulze-Lefert, P. (2004). RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. Plant Cell 16, 3480–3495. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

