Functional architecture of retinotopy in visual association cortex of behaving monkey
- PMID: 15749989
- PMCID: PMC1945172
- DOI: 10.1093/cercor/bhh148
Functional architecture of retinotopy in visual association cortex of behaving monkey
Abstract
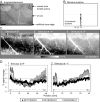
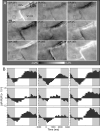
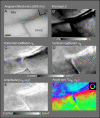
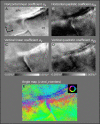
While the receptive field properties of single neurons in the inferior parietal cortex have been quantitatively described from numerous electrical measurements, the visual topography of area 7a and the adjacent dorsal prelunate area (DP) remains unknown. This lacuna may be a technical byproduct of the difficulty of reconstructing tens to hundreds of penetrations, or may be the result of varying functional retinotopic architectures. Intrinsic optical imaging, performed in behaving monkey for extended periods of time, was used to evaluate retinotopy simultaneously at multiple positions across the cortical surface. As electrical recordings through an implanted artificial dura are difficult, the measurement and quantification of retinotopy with long-term recordings was validated by imaging early visual cortex (areas V1 and V2). Retinotopic topography was found in each of the three other areas studied within a single day's experiment. However, the ventral portion of DP (DPv) had a retinotopic topography that varied from day to day, while the more dorsal aspects (DPd) exhibited consistent retinotopy. This suggests that the dorsal prelunate gyrus may consist of more than one visual area. The retinotopy of area 7a also varied from day to day. Possible mechanisms for this variability across days are discussed as well as its impact upon our understanding of the representation of extrapersonal space in the inferior parietal cortex.
Figures







References
-
- Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–723.
-
- Albright TD, Stoner GR. Contextual influence on visual processing. Annu Rev Neurosci. 2002;25:339–379. - PubMed
-
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparison in visual neurons. Annu Rev Neurosci. 1985;8:407–430. - PubMed
-
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. - PubMed
-
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230:456–458. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

