Paracetamol: a haemorrhagic risk factor in patients on warfarin
- PMID: 15752384
- PMCID: PMC1884780
- DOI: 10.1111/j.1365-2125.2004.02199.x
Paracetamol: a haemorrhagic risk factor in patients on warfarin
Abstract
Aim: To quantify the effect of paracetamol on the anticoagulant effect of warfarin under normal clinical conditions.
Patients and methods: In a prospective double-blind, cross-over, placebo-controlled study, 11 patients on stable warfarin therapy received in random order two 14-day regimens of paracetamol 4 g day(-1) or placebo, with a 14-day or more wash-out period in between, time necessary to fulfil the inclusion criteria.
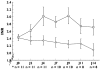
Results: In patients on paracetamol, the mean maximum increase in the International Normalized Ratio (INR) observed was 1.04 +/- 0.55 vs. 0.20 +/- 0.32 in those on placebo (P = 0.003). The mean maximum INR observed was significantly higher with paracetamol than with placebo (3.47 vs. 2.61, P = 0.01). In patients receiving paracetamol, the mean observed INR was significantly increased after 4 days (+ 0.6 +/- 0.6, P < 0.001).
Conclusion: Paracetamol at 4 g day(-1) induces a significant increase in INR in patients receiving a stable regimen of warfarin, increasing the risk of bleeding associated with warfarin.
Figures

References
-
- Hirsch J, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119:8S–21S. - PubMed
-
- Levine MN, Raskob G, Landefeld S, Kearon C. Haemorrhagic complications of anticoagulant treatment. Chest. 2001;119:108S–121S. - PubMed
-
- American College of Rheumatology Subcommittee on osteoarthritis guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee 2000 update. Arthritis Rheum. 2000;43:1905–15. - PubMed
-
- Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE. Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA. 1998;279:657–62. - PubMed
-
- Mahé I, Caulin C, Bergmann JF. Does paracetamol (acetaminophen) potentiate the effects of oral anticoagulants. A literature review. Drug Safety. 2004;27:283–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

