Thermodynamic and structural characterization of amino acid-linked dialkyl lipids
- PMID: 15752461
- PMCID: PMC2720572
- DOI: 10.1016/j.chemphyslip.2004.11.001
Thermodynamic and structural characterization of amino acid-linked dialkyl lipids
Abstract
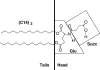
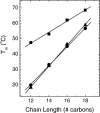
Using differential scanning calorimetry (DSC), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR), we determined some thermodynamic and structural parameters for a series of amino acid-linked dialkyl lipids containing a glutamic acid-succinate headgroup and di-alkyl chains: C12, C14, C16 and C18 in CHES buffer, pH 10. Upon heating, DSC shows that the C12, C14 and annealed C16 lipids undergo a single transition which XRD shows is from a lamellar, chain ordered subgel phase to a fluid phase. This single transition splits into two transitions for C18, and FTIR shows that the upper main transition is predominantly the melting of the hydrocarbon chains whereas the lower transition involves changes in the headgroup ordering as well as changes in the lateral packing of the chains. For short incubation times at low temperature, the C16 lipid appears to behave like the C18 lipid, but appropriate annealing at low temperatures indicates that its true equilibrium behavior is like the shorter chain lipids. XRD shows that the C12 lipid readily converts into a highly ordered subgel phase upon cooling and suggests a model with untilted, interdigitated chains and an area of 77.2A(2)/4 chains, with a distorted orthorhombic unit subcell, a=9.0A, b=4.3A and beta=92.7 degrees . As the chain length n increases, subgel formation is slowed, but untilted, interdigitated chains prevail.
Figures







Similar articles
-
The physical properties of glycosyl diacylglycerols. Calorimetric, X-ray diffraction and Fourier transform spectroscopic studies of a homologous series of 1,2-di-O-acyl-3-O-(beta-D-galactopyranosyl)-sn-glycerols.Chem Phys Lipids. 2001 Jun;111(2):139-61. doi: 10.1016/s0009-3084(01)00153-0. Chem Phys Lipids. 2001. PMID: 11457442
-
The thermotropic phase behaviour and phase structure of a homologous series of racemic beta-D-galactosyl dialkylglycerols studied by differential scanning calorimetry and X-ray diffraction.Chem Phys Lipids. 2007 Jul;148(1):26-50. doi: 10.1016/j.chemphyslip.2007.04.004. Epub 2007 Apr 19. Chem Phys Lipids. 2007. PMID: 17524381
-
Calorimetric, x-ray diffraction, and spectroscopic studies of the thermotropic phase behavior and organization of tetramyristoyl cardiolipin membranes.Biophys J. 2007 May 1;92(9):3166-77. doi: 10.1529/biophysj.106.094003. Epub 2007 Feb 9. Biophys J. 2007. PMID: 17293402 Free PMC article.
-
The thermotropic phase behavior of cationic lipids: calorimetric, infrared spectroscopic and X-ray diffraction studies of lipid bilayer membranes composed of 1,2-di-O-myristoyl-3-N,N,N-trimethylaminopropane (DM-TAP).Biochim Biophys Acta. 2001 Feb 9;1510(1-2):70-82. doi: 10.1016/s0005-2736(00)00336-9. Biochim Biophys Acta. 2001. PMID: 11342148
-
The interfacial structure of phospholipid bilayers: differential scanning calorimetry and Fourier transform infrared spectroscopic studies of 1,2-dipalmitoyl-sn-glycero-3-phosphorylcholine and its dialkyl and acyl-alkyl analogs.Biophys J. 1996 Jun;70(6):2736-46. doi: 10.1016/S0006-3495(96)79843-0. Biophys J. 1996. PMID: 8744311 Free PMC article.
Cited by
-
Structure and thermotropic behavior of the Staphylococcus aureus lipid lysyl-dipalmitoylphosphatidylglycerol.Biophys J. 2008 Mar 15;94(6):2150-9. doi: 10.1529/biophysj.107.123422. Epub 2007 Nov 30. Biophys J. 2008. PMID: 18055539 Free PMC article.
-
On the propensity of phosphatidylglycerols to form interdigitated phases.Biophys J. 2007 Jul 15;93(2):513-25. doi: 10.1529/biophysj.106.101592. Epub 2007 Apr 20. Biophys J. 2007. PMID: 17449673 Free PMC article.
References
-
- Aoki H, Koto T, Kodama M. Calorimetric investigation of conversion to the most stable subgel phase of phosphatidylethanolamine-water system. J. Thermal Anal. Calorim. 2001;64:299–306.
-
- Berndt P, Fields G, Tirrell M. Synthetic lipidation of peptides and amino acids—monolayer structure and properties. J. Am. Chem. Soc. 1995a;117:9515–9522.
-
- Berndt P, Kurihara K, Kunitake T. Measurement of forces between surfaces composed of two-dimensionally oriented, complementary and noncomplementary nucleobases. Langmuir. 1995b;11:3083–3091.
-
- Boggs JM. Intermolecular hydrogen bonding between lipids: influence on organization and function of lipids in membranes. Can. J. Biochem. 1980;58:755–770. - PubMed
-
- Dori Y, Bianco-Peled H, Satija S, Fields GB, McCarthy JB, Tirrell M. Ligand accessibility as a means to control cell response to bioactive bilayer membranes. J. Biomed. Mater. Res. 2000;50:75–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

