Heterologous T cell immunity in severe hepatitis C virus infection
- PMID: 15753202
- PMCID: PMC2212827
- DOI: 10.1084/jem.20041058
Heterologous T cell immunity in severe hepatitis C virus infection
Abstract
Hepatitis C virus (HCV) can cause liver disease of variable severity. Expansion of preexisting memory CD8 T cells by cross-reactivity with a new heterologous virus infection has been shown in mice to shape the repertoire of the primary response and to influence virus-related immunopathology. To determine whether this mechanism can influence the course of HCV infection, we analyzed the features of the HCV-specific CD8 T cell response in eight patients with acute HCV infection, two of whom had a particularly severe illness. Patients with severe hepatitis, but not those with mild disease, showed an extremely vigorous CD8 T cell response narrowly focused on a single epitope (NS3 1073-1081), which cross-reacted with an influenza neuraminidase sequence. Our results suggest that CD8 T cell cross-reactivity influences the severity of the HCV-associated liver pathology and depicts a model of disease induction that may apply to different viral infections.
Figures



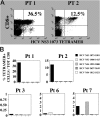


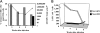
References
-
- Farci, P., H.J. Alter, A. Shimoda, S. Govindarajan, L.C. Cheung, J.C. Melpolder, R.A. Sacher, J.W. Shih, and R.H. Purcell. 1996. Hepatitis C virus-associated fulminant hepatic failure. N. Engl. J. Med. 335:631–634. - PubMed
-
- SEIEVA Collaborating Group. 2003. Case fatality rate of acute viral hepatitis in Italy: 1995-2000. An update. Dig. Liver Dis. 35:404–408. - PubMed
-
- Welsh, R.M., and L.K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417–426. - PubMed
-
- Brehm, M.A., A.K. Pinto, K.A. Daniels, J.P. Schneck, R.M. Welsh, and L.K. Selin. 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3:627–634. - PubMed
-
- Chen, H.D., A.E. Fraire, I. Joris, M.A. Brehm, R.M. Welsh, and L.K. Selin. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2:1067–1076. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

