Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis
- PMID: 15753209
- PMCID: PMC2212832
- DOI: 10.1084/jem.20040429
Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis
Erratum in
- J Exp Med. 2005 Sep 5;202(5):721
Abstract
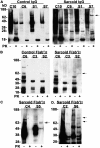
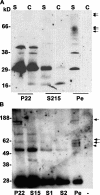
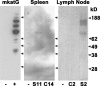
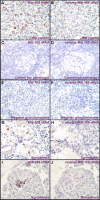
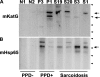
Sarcoidosis is a disease of unknown etiology characterized by noncaseating epithelioid granulomas, oligoclonal CD4(+) T cell infiltrates, and immune complex formation. To identify pathogenic antigens relevant to immune-mediated granulomatous inflammation in sarcoidosis, we used a limited proteomics approach to detect tissue antigens that were poorly soluble in neutral detergent and resistant to protease digestion, consistent with the known biochemical properties of granuloma-inducing sarcoidosis tissue extracts. Tissue antigens with these characteristics were detected with immunoglobulin (Ig)G or F(ab')(2) fragments from the sera of sarcoidosis patients in 9 of 12 (75%) sarcoidosis tissues (150-160, 80, or 60-64 kD) but only 3 of 22 (14%) control tissues (all 62-64 kD; P = 0.0006). Matrix-assisted laser desorption/ionization time of flight mass spectrometry identified Mycobacterium tuberculosis catalase-peroxidase (mKatG) as one of these tissue antigens. Protein immunoblotting using anti-mKatG monoclonal antibodies independently confirmed the presence of mKatG in 5 of 9 (55%) sarcoidosis tissues but in none of 14 control tissues (P = 0.0037). IgG antibodies to recombinant mKatG were detected in the sera of 12 of 25 (48%) sarcoidosis patients compared with 0 of 11 (0%) purified protein derivative (PPD)(-) (P = 0.0059) and 4 of 10 (40%) PPD(+) (P = 0.7233) control subjects, suggesting that remnant mycobacterial catalase-peroxidase is one target of the adaptive immune response driving granulomatous inflammation in sarcoidosis.
Figures

Similar articles
-
T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis.J Immunol. 2008 Dec 15;181(12):8784-96. doi: 10.4049/jimmunol.181.12.8784. J Immunol. 2008. PMID: 19050300 Free PMC article.
-
Potential etiologic agents in sarcoidosis.Proc Am Thorac Soc. 2007 Aug 15;4(5):465-8. doi: 10.1513/pats.200608-155MS. Proc Am Thorac Soc. 2007. PMID: 17684291 Free PMC article. Review.
-
Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis.Infect Immun. 2007 Jan;75(1):527-30. doi: 10.1128/IAI.00732-06. Epub 2006 Nov 6. Infect Immun. 2007. PMID: 17088357 Free PMC article.
-
Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL.J Clin Immunol. 2012 Oct;32(5):1129-40. doi: 10.1007/s10875-012-9700-5. Epub 2012 May 3. J Clin Immunol. 2012. PMID: 22552860 Free PMC article.
-
Evidence for mycobacteria in sarcoidosis.Am J Respir Cell Mol Biol. 2011 Nov;45(5):899-905. doi: 10.1165/rcmb.2010-0433TR. Epub 2011 Jun 9. Am J Respir Cell Mol Biol. 2011. PMID: 21659662 Free PMC article. Review.
Cited by
-
Involvement of Dendritic Cells and Th17 Cells in Induced Tertiary Lymphoid Structures in a Chronic Beryllium Disease Mouse Model.Mediators Inflamm. 2021 May 6;2021:8845966. doi: 10.1155/2021/8845966. eCollection 2021. Mediators Inflamm. 2021. PMID: 34054347 Free PMC article.
-
Mycobacterium tuberculosis Antigen 85A induces Th-1 immune responses in systemic sarcoidosis.J Clin Immunol. 2007 Jul;27(4):445-54. doi: 10.1007/s10875-007-9080-4. Epub 2007 Mar 15. J Clin Immunol. 2007. PMID: 17357846 Free PMC article.
-
Expression profiling in granulomatous lung disease.Proc Am Thorac Soc. 2007 Jan;4(1):101-7. doi: 10.1513/pats.200607-140JG. Proc Am Thorac Soc. 2007. PMID: 17202298 Free PMC article. Review.
-
No evidence of altered alveolar macrophage polarization, but reduced expression of TLR2, in bronchoalveolar lavage cells in sarcoidosis.Respir Res. 2010 Sep 2;11(1):121. doi: 10.1186/1465-9921-11-121. Respir Res. 2010. PMID: 20813038 Free PMC article.
-
A concise review of pulmonary sarcoidosis.Am J Respir Crit Care Med. 2011 Mar 1;183(5):573-81. doi: 10.1164/rccm.201006-0865CI. Epub 2010 Oct 29. Am J Respir Crit Care Med. 2011. PMID: 21037016 Free PMC article. Review.
References
-
- Statement on sarcoidosis. 1999. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 160:736–755. - PubMed
-
- Moller, D.R., J.D. Forman, M.C. Liu, P.W. Noble, B.M. Greenlee, P. Vyas, D.A. Holden, J.M. Forrester, A. Lazarus, M. Wysocka, and G. Trinchieri. 1996. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J. Immunol. 145: 4952–4960. - PubMed
-
- Greene, C.M., G. Meachery, C.C. Taggart, C.P. Rooney, R. Coakley, S.J. O'Neill, and N.G. McElvaney. 2000. Role of IL-18 in CD4+ T lymphocyte activation in sarcoidosis. J. Immunol. 165:4718–4724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

