Differential use of endoplasmic reticulum membrane for phagocytosis in J774 macrophages
- PMID: 15753287
- PMCID: PMC554806
- DOI: 10.1073/pnas.0409219102
Differential use of endoplasmic reticulum membrane for phagocytosis in J774 macrophages
Abstract
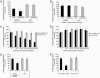
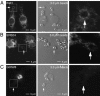
Sustained phagocytosis requires the continuous replacement of cell-surface membrane from intracellular sources. Depending on the nature of the engulfed particles, a variety of endocytic compartments have been demonstrated to contribute membranes needed for the formation of phagosomes. It has recently been reported that the endoplasmic reticulum (ER) can also fuse with the plasma membrane during phagocytosis [Gagnon, E., Duclos, S., Rondeau, C., Chevet, E., Cameron, P. H., Steele-Mortimer, O., Paiement, J., Bergeron, J. J. & Desjardins, M. (2002) Cell 110, 119-131]. However, there is currently no known mechanistic basis for this fusion process to occur. Here we report that direct ER-plasma membrane fusion during phagocytosis requires the ER resident soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein ERS24/Sec22b and that J774-macrophages react toward the challenge of large (3.0-microm) but not small (0.8-microm) particles by triggering this fusion mechanism, allowing them to access the most abundant endogenous membrane source in the cell, the ER.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

