Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector
- PMID: 15753292
- PMCID: PMC554836
- DOI: 10.1073/pnas.0500930102
Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector
Abstract
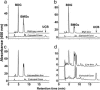
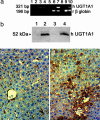
Crigler-Najjar syndrome is a recessively inherited disorder characterized by severe unconjugated hyperbilirubinemia caused by a deficiency of uridine diphospho-glucuronosyl transferase 1A1. Current therapy relies on phototherapy to prevent kernicterus, but liver transplantation presently is the only permanent cure. Gene therapy is a potential alternative, and recent work has shown that helper-dependent adenoviral (HD-Ad) vectors, devoid of all viral coding sequences, induce prolonged transgene expression and exhibit significantly less chronic toxicity than early-generation Ad vectors. We used a HD-Ad vector to achieve liver-restricted expression of human uridine diphospho-glucuronosyl transferase 1A1 in the Gunn rat, a model of the human disorder. Total plasma bilirubin levels were reduced from >5.0 mg/dl to <<1.4 mg/dl for >2 yr after a single i.v. administration of vector expressing the therapeutic transgene at a dose of 3 x 10(12) viral particles per kg. HPLC analysis of bile from treated rats showed the presence of bilirubin glucuronides at normal WT levels >2 yr after one injection of vector, and i.v. injection of bilirubins IIIalpha and XIIIalpha in the same animals revealed excess bilirubin-conjugating capacity. There was no significant elevation of liver enzymes (alanine aminotransferase) and only transient, moderate thrombocytopenia after injection of the vector. A clinically significant reduction in serum bilirubin was observed with a dose as low as 6 x 10(11) viral particles per kg. We conclude that complete, long-term correction of hyperbilirubinemia in the Gunn rat model of Crigler-Najjar syndrome can be achieved with one injection of HD-Ad vector and negligible chronic toxicity.
Figures


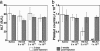
References
-
- Schmid, R. & McDonagh, A. F. (1978) in The Metabolic Basis of Inherited Diseases, eds. Stanbury, J. B., Wyngaarden, J. B. & Fredrickson, D. S. (McGraw–Hill, New York), pp. 1221–1257.
-
- Ciotti, M., Obaray, R., Martin, M. G. & Owens, I. S. (1997) Am. J. Med. Genet. 68, 173–178. - PubMed
-
- van der Veere, C. N., Sinaasappel, M., McDonagh, A. F., Rosenthal, P., Labrune, P., Odievre, M., Fevery, J., Otte, J. B., McClean, P., Burk, G., et al. (1996) Hepatology 24, 311–315. - PubMed
-
- Fox, I. J., Chowdhury, J. R., Kaufman, S. S., Goertzen, T. C., Chowdhury, N. R., Warkentin, P. I., Dorko, K., Sauter, B. V. & Strom, S. C. (1998) N. Engl. J. Med. 338, 1422–1426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

