The protooncogene MYC can break B cell tolerance
- PMID: 15753301
- PMCID: PMC552974
- DOI: 10.1073/pnas.0409832102
The protooncogene MYC can break B cell tolerance
Abstract
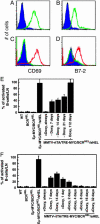
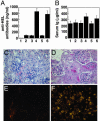
The protooncogene MYC has been implicated in both the proliferation and programmed cell death of lymphoid cells, and in the genesis of lymphoid tumors. Here, we report that overexpression of MYC, as found in many lymphomas, can break immune tolerance. Mice that would otherwise be tolerant to a transgenic autoantigen mounted an immune response to the antigen if MYC was vigorously expressed in the B cell lineage. The responsive B cells converted to an activated phenotype and produced copious amounts of autoantibody that engendered immune complex disease of the kidney. MYC was required to both establish and maintain the breach of tolerance. These effects may be due to the ability of MYC to serve as a surrogate for cytokines. We found that the gene could mimic the effects of cytokines on both B cell proliferation and survival and, indeed, was required for those effects. These findings demonstrate a critical role for MYC in the response of B cells to antigen and expand the potential contributions of MYC to the genesis of lymphomas.
Figures



Similar articles
-
Understanding B-cell tolerance through the use of immunoglobulin transgenic models.Immunol Res. 2008;40(3):208-23. doi: 10.1007/s12026-007-8008-7. Immunol Res. 2008. PMID: 17943232 Review.
-
CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis.J Clin Invest. 2012 Jun;122(6):2257-66. doi: 10.1172/JCI45851. Epub 2012 May 1. J Clin Invest. 2012. PMID: 22546857 Free PMC article.
-
B-cell activating factor and v-Myc myelocytomatosis viral oncogene homolog (c-Myc) influence progression of chronic lymphocytic leukemia.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18956-60. doi: 10.1073/pnas.1013420107. Epub 2010 Oct 18. Proc Natl Acad Sci U S A. 2010. PMID: 20956327 Free PMC article.
-
Histone H2AX suppresses translocations in lymphomas of Eμ-c-Myc transgenic mice that contain a germline amplicon of tumor-promoting genes.Cell Cycle. 2013 Sep 1;12(17):2867-75. doi: 10.4161/cc.25922. Epub 2013 Aug 8. Cell Cycle. 2013. PMID: 23966158 Free PMC article.
-
B cell conducts the lymphocyte orchestra.J Autoimmun. 2007 Mar-May;28(2-3):143-51. doi: 10.1016/j.jaut.2007.02.011. Epub 2007 Mar 23. J Autoimmun. 2007. PMID: 17363215 Review.
Cited by
-
The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas.PLoS Biol. 2008 Jun 24;6(6):e152. doi: 10.1371/journal.pbio.0060152. PLoS Biol. 2008. PMID: 18578569 Free PMC article.
-
Understanding B-cell tolerance through the use of immunoglobulin transgenic models.Immunol Res. 2008;40(3):208-23. doi: 10.1007/s12026-007-8008-7. Immunol Res. 2008. PMID: 17943232 Review.
-
Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target.Blood. 2009 Mar 12;113(11):2508-16. doi: 10.1182/blood-2008-05-158618. Epub 2008 Nov 3. Blood. 2009. PMID: 18981293 Free PMC article.
-
SPLUNC1 regulation in airway epithelial cells: role of Toll-like receptor 2 signaling.Respir Res. 2010 Nov 5;11(1):155. doi: 10.1186/1465-9921-11-155. Respir Res. 2010. PMID: 21054862 Free PMC article.
-
The transcription factor Bright plays a role in marginal zone B lymphocyte development and autoantibody production.Mol Immunol. 2011 Oct;49(1-2):367-79. doi: 10.1016/j.molimm.2011.09.008. Epub 2011 Oct 2. Mol Immunol. 2011. PMID: 21963220 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

