Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion
- PMID: 15753306
- PMCID: PMC554795
- DOI: 10.1073/pnas.0408679102
Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion
Abstract
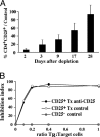
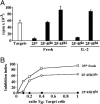
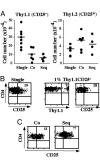
Expression of the IL-2 receptor alpha chain (CD25) by peripheral CD4 T cells follows cellular activation. However, CD25 expression by CD4 cells is widely used as a marker to identify regulatory T cells (T(R)), although cells with regulatory properties are also found in the CD4+CD25- subset. By using in vivo functional assays and Foxp3 expression as a faithful marker of T(R) differentiation, we have evaluated the requirements for CD25 expression by peripheral T(R). We first show that in vivo depletion of CD25+ cells prevents the development of spontaneous encephalomyelitis in recombination-activating gene (RAG)-deficient anti-myelin basic protein T cell antigen receptor (TCR) transgenic mice, and allows disease induction in otherwise healthy RAG-competent transgenic mice. Similar treatment in normal thymectomized animals is followed by the fast recovery of a normal number of CD25+ T(R). Consistently, Foxp3-expressing T(R) encompassed in the CD25- cell population convert to CD25+ after homeostatic expansion and are selectable by IL-2 in vitro. Surface expression of CD25 on T(R) is controlled by the activity of conventional CD4 cells and is fully labile because it can be lost and regained without affecting the functional potential of the cells. These findings reveal that Foxp3-expressing CD25- cells constitute a peripheral reservoir of differentiated T(R), recruited to the CD25+ pool upon homeostatic expansion and/or activation. This analysis, together with the notion that physiological commitment of T(R) takes place exclusively in the thymus should help for the interpretation of experiments assessing peripheral T(R) differentiation from naive CD4 T cells, defined as CD25-.
Figures
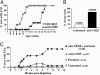


Similar articles
-
Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization.J Exp Med. 2005 Mar 7;201(5):723-35. doi: 10.1084/jem.20041982. J Exp Med. 2005. PMID: 15753206 Free PMC article.
-
Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells.Int Immunol. 2004 Aug;16(8):1189-201. doi: 10.1093/intimm/dxh122. Epub 2004 Jul 5. Int Immunol. 2004. PMID: 15237110
-
Foxp3 programs the development and function of CD4+CD25+ regulatory T cells.Nat Immunol. 2003 Apr;4(4):330-6. doi: 10.1038/ni904. Epub 2003 Mar 3. Nat Immunol. 2003. PMID: 12612578
-
Control of T-cell activation by CD4+ CD25+ suppressor T cells.Immunol Rev. 2001 Aug;182:58-67. doi: 10.1034/j.1600-065x.2001.1820104.x. Immunol Rev. 2001. PMID: 11722623 Review.
-
Human CD4+CD25+ regulatory T cells.Semin Immunol. 2004 Apr;16(2):89-98. doi: 10.1016/j.smim.2003.12.005. Semin Immunol. 2004. PMID: 15036232 Review.
Cited by
-
Regulatory T cells in experimental autoimmune disease.Springer Semin Immunopathol. 2006 Aug;28(1):3-16. doi: 10.1007/s00281-006-0021-8. Epub 2006 Jul 13. Springer Semin Immunopathol. 2006. PMID: 16838180 Review.
-
Increase in tumour-infiltrating lymphocytes with regulatory T cell immunophenotypes and reduced zeta-chain expression in nasopharyngeal carcinoma patients.Clin Exp Immunol. 2009 Mar;155(3):412-22. doi: 10.1111/j.1365-2249.2008.03793.x. Clin Exp Immunol. 2009. PMID: 19220831 Free PMC article.
-
Depletion of CD25⁺ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma.Blood. 2011 Jun 23;117(25):6952-62. doi: 10.1182/blood-2010-12-326108. Epub 2011 Apr 26. Blood. 2011. PMID: 21521781 Free PMC article.
-
Expression of TIM-3, Human β-defensin-2, and FOXP3 and Correlation with Disease Activity in Pediatric Crohn's Disease with Infliximab Therapy.Gut Liver. 2015 May 23;9(3):370-80. doi: 10.5009/gnl13408. Gut Liver. 2015. PMID: 25071071 Free PMC article.
-
Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system.Aging Cell. 2016 Feb;15(1):28-38. doi: 10.1111/acel.12405. Epub 2015 Oct 13. Aging Cell. 2016. PMID: 26463117 Free PMC article.
References
-
- Coutinho, A., Kazatchkine, M. D. & Avrameas, S. (1995) Curr. Opin. Immunol. 7, 812–818. - PubMed
-
- Lee, W. T., Yin, X. M. & Vitetta, E. S. (1990) J. Immunol. 144, 3288–3295. - PubMed
-
- Powrie, F., Leach, M. W., Mauze, S., Caddle, L. B. & Coffman, R. L. (1993) Int. Immunol. 5, 1461–1471. - PubMed
-
- Annacker, O., Burlen-Defranoux, O., Pimenta-Araujo, R., Cumano, A. & Bandeira, A. (2000) J. Immunol. 164, 3573–3580. - PubMed
-
- Mittrucker, H. W. & Kaufmann, S. H. (2004) Eur. J. Immunol. 34, 306–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

