Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals
- PMID: 15753314
- PMCID: PMC554796
- DOI: 10.1073/pnas.0500146102
Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals
Abstract
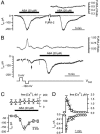
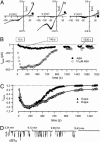
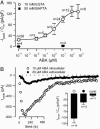
The phytohormone abscisic acid (ABA) reports on the water status of the plant and induces stomatal closure. Guard cell anion channels play a central role in this response, because they mediate anion efflux, and in turn, cause a depolarization-induced K+ release. We recorded early steps in ABA signaling, introducing multibarreled microelectrodes in guard cells of intact plants. Upon external ABA treatment, anion channels transiently activated after a lag phase of approximately 2 min. As expected for a cytosolic ABA receptor, iontophoretic ABA loading into the cytoplasm initiated a rise in anion current without delay. These ABA responses could be elicited repetitively at resting and at largely depolarized potentials (e.g., 0 mV), ruling out signal transduction by means of hyperpolarization-activated calcium channels. Likewise, ABA stimulation did not induce a rise in the cytosolic free-calcium concentration. However, the presence of approximately 100 nM background Ca2+ was required for anion channel function, because the action of ABA on anion channels was repressed after loading of the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetate. The chain of events appears very direct, because none of the tested putative ABA-signaling intermediates (inositol 1,4,5 trisphosphate, inositol hexakisphosphate, nicotinic acid adenine dinucleotide phosphate, and cyclic ADP-ribose), could mimic ABA as anion channel activator. In patch-clamp experiments, cytosolic ABA also evoked anion current transients carried by R- and S-type anion channels. The response was dose-dependent with half-maximum activation at 2.6 microM ABA. Our studies point to an ABA pathway initiated by ABA binding to a cytosolic receptor that within seconds activates anion channels, and in turn, leads to depolarization of the plasma membrane.
Figures


Similar articles
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
-
Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels.Proc Natl Acad Sci U S A. 1990 Dec;87(23):9305-9. doi: 10.1073/pnas.87.23.9305. Proc Natl Acad Sci U S A. 1990. PMID: 2174559 Free PMC article.
-
Ca2+-dependent and -independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum.Plant Physiol. 2007 Jan;143(1):28-37. doi: 10.1104/pp.106.092643. Epub 2006 Dec 1. Plant Physiol. 2007. PMID: 17142476 Free PMC article.
-
Dual role of cytosolic GSH in the ABA signaling pathway and plasma membrane ion channel regulation in guard cells of Vicia faba.J Plant Physiol. 2025 Mar;306:154447. doi: 10.1016/j.jplph.2025.154447. Epub 2025 Feb 5. J Plant Physiol. 2025. PMID: 39923261
-
A new scheme of symbiosis: ligand- and voltage-gated anion channels in plants and animals.Philos Trans R Soc Lond B Biol Sci. 1992 Oct 29;338(1283):31-8. doi: 10.1098/rstb.1992.0126. Philos Trans R Soc Lond B Biol Sci. 1992. PMID: 1280838 Review.
Cited by
-
The Clickable Guard Cell, Version II: Interactive Model of Guard Cell Signal Transduction Mechanisms and Pathways.Arabidopsis Book. 2008;6:e0114. doi: 10.1199/tab.0114. Epub 2008 Nov 26. Arabidopsis Book. 2008. PMID: 22303239 Free PMC article.
-
Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions.Genes Dev. 2010 Aug 15;24(16):1695-708. doi: 10.1101/gad.1953910. Genes Dev. 2010. PMID: 20713515 Free PMC article. Review.
-
Mechanisms of abscisic acid-mediated control of stomatal aperture.Curr Opin Plant Biol. 2015 Dec;28:154-62. doi: 10.1016/j.pbi.2015.10.010. Epub 2015 Nov 19. Curr Opin Plant Biol. 2015. PMID: 26599955 Free PMC article. Review.
-
Optogenetic control of the guard cell membrane potential and stomatal movement by the light-gated anion channel GtACR1.Sci Adv. 2021 Jul 9;7(28):eabg4619. doi: 10.1126/sciadv.abg4619. Print 2021 Jul. Sci Adv. 2021. PMID: 34244145 Free PMC article.
-
Exploring emergent properties in cellular homeostasis using OnGuard to model K+ and other ion transport in guard cells.J Plant Physiol. 2014 May 15;171(9):770-8. doi: 10.1016/j.jplph.2013.09.014. Epub 2013 Nov 21. J Plant Physiol. 2014. PMID: 24268743 Free PMC article. Review.
References
-
- Chinnusamy, V., Schumaker, K. & Zhu, J. K. (2004) J. Exp. Bot. 55, 225–236. - PubMed
-
- Raschke, K., Hedrich, R., Reckmann, U. & Schroeder, J. I. (1988) Bot. Acta 101, 283–294.
-
- MacRobbie, E. A. C. (1987) in Stomatal Function, eds. Zeiger, E., Farquhar, G. D. & Cowan I. R. (Stanford Univ. Press, Stanford CA), pp. 125–162.
-
- Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waren, D. (2001) Ann. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

