Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals
- PMID: 15753314
- PMCID: PMC554796
- DOI: 10.1073/pnas.0500146102
Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals
Abstract
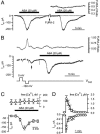
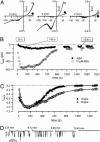
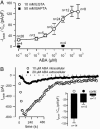
The phytohormone abscisic acid (ABA) reports on the water status of the plant and induces stomatal closure. Guard cell anion channels play a central role in this response, because they mediate anion efflux, and in turn, cause a depolarization-induced K+ release. We recorded early steps in ABA signaling, introducing multibarreled microelectrodes in guard cells of intact plants. Upon external ABA treatment, anion channels transiently activated after a lag phase of approximately 2 min. As expected for a cytosolic ABA receptor, iontophoretic ABA loading into the cytoplasm initiated a rise in anion current without delay. These ABA responses could be elicited repetitively at resting and at largely depolarized potentials (e.g., 0 mV), ruling out signal transduction by means of hyperpolarization-activated calcium channels. Likewise, ABA stimulation did not induce a rise in the cytosolic free-calcium concentration. However, the presence of approximately 100 nM background Ca2+ was required for anion channel function, because the action of ABA on anion channels was repressed after loading of the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetate. The chain of events appears very direct, because none of the tested putative ABA-signaling intermediates (inositol 1,4,5 trisphosphate, inositol hexakisphosphate, nicotinic acid adenine dinucleotide phosphate, and cyclic ADP-ribose), could mimic ABA as anion channel activator. In patch-clamp experiments, cytosolic ABA also evoked anion current transients carried by R- and S-type anion channels. The response was dose-dependent with half-maximum activation at 2.6 microM ABA. Our studies point to an ABA pathway initiated by ABA binding to a cytosolic receptor that within seconds activates anion channels, and in turn, leads to depolarization of the plasma membrane.
Figures


References
-
- Chinnusamy, V., Schumaker, K. & Zhu, J. K. (2004) J. Exp. Bot. 55, 225–236. - PubMed
-
- Raschke, K., Hedrich, R., Reckmann, U. & Schroeder, J. I. (1988) Bot. Acta 101, 283–294.
-
- MacRobbie, E. A. C. (1987) in Stomatal Function, eds. Zeiger, E., Farquhar, G. D. & Cowan I. R. (Stanford Univ. Press, Stanford CA), pp. 125–162.
-
- Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waren, D. (2001) Ann. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

