Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis
- PMID: 15753315
- PMCID: PMC554822
- DOI: 10.1073/pnas.0409716102
Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis
Abstract
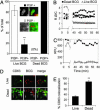
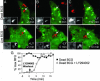
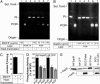
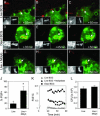
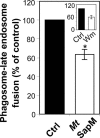
Live Mycobacterium tuberculosis persists in macrophage phagosomes by interfering with phagolysosome biogenesis. Here, using four-dimensional microscopy and in vitro assays, we report the principal difference between phagosomes containing live and dead mycobacteria. Phosphatidylinositol 3-phosphate (PI3P), a membrane trafficking regulatory lipid essential for phagosomal acquisition of lysosomal constituents, is retained on phagosomes harboring dead mycobacteria but is continuously eliminated from phagosomes with live bacilli. We show that the exclusion of PI3P from live mycobacterial phagosomes can be only transiently reversed by Ca2+ fluxes, and that live M. tuberculosis secretes a lipid phosphatase, SapM, that hydrolyzes PI3P, inhibits phagosome-late endosome fusion in vitro, and contributes to inhibition of phagosomal maturation.
Figures
References
-
- Vergne, I., Chua, J., Singh, S. B. & Deretic, V. (2004) Annu. Rev. Cell Dev. Biol. 20, 367–394. - PubMed
-
- Sturgill-Koszycki, S., Schlesinger, P. H., Chakraborty, P., Haddix, P. L., Collins, H. L., Fok, A. K., Allen, R. D., Gluck, S. L., Heuser, J. & Russell, D. G. (1994) Science 263, 678–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

