Homeostatic regulation of zinc transporters in the human small intestine by dietary zinc supplementation
- PMID: 15753530
- PMCID: PMC1774437
- DOI: 10.1136/gut.2004.041962
Homeostatic regulation of zinc transporters in the human small intestine by dietary zinc supplementation
Abstract
Background: The role of intestinal transporter regulation in optimising nutrient absorption has been studied extensively in rodent and cell line models but not in human subjects.
Aims: The aim of the present study was to investigate the response in vivo of zinc transporters in the human enterocyte to dietary zinc supplementation.
Subjects: Eighteen patients who had previously undergone ileostomy, all free of any symptoms of inflammatory bowel disease.
Methods: Subjects took a daily zinc supplement of 25 mg for 14 days in a double blind, placebo controlled, crossover trial. The effect of the supplement on expression in ileal biopsies of the zinc transporters SLC30A1, SLC30A4, SLC30A5, SLC39A1, SLC39A4, and metallothionein was measured by reverse transcription-polymerase chain reaction RT-PCR. Expression of SLC30A1, SLC30A5, and SLC39A4 was also examined by immunoblotting.
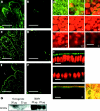
Results: The zinc supplement reduced SLC30A1 mRNA (1.4-fold) together with SLC30A1, SLC30A5, and SLC39A4 protein (1.8-fold, 3.7-fold, and to undetectable levels, respectively) in ileal mucosa and increased metallothionein mRNA (1.7-fold). The supplement had no effect on expression of SLC30A4 or SLC39A1 mRNA. Localisation of SLC30A5 at the apical human enterocyte/colonocyte membrane and also at the apical membrane of Caco-2 cells was demonstrated by immunohistochemistry. Commensurate with these observations in zinc supplemented human subjects, SLC30A1, SLC30A5, and SLC39A4 mRNA and protein were reduced in Caco-2 cells cultured at 200 muM compared with 100 muM zinc.
Conclusions: These observations indicate that, in response to variations in dietary zinc intakes, regulated expression of plasma membrane zinc transporters in the human intestine contributes to maintenance of zinc status.
Figures



References
-
- Hirst BH. Dietary regulation of intestinal nutrient carriers. Proc Nutr Soc 1993;52:315–24. - PubMed
-
- Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev 1997;77:257–302. - PubMed
-
- Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997;388:482–8. - PubMed
-
- Cragg RA, Christie GR, Phillips SR, et al. A novel zinc-regulated human zinc transporter, SLC30A5, is localised to the enterocyte apical membrane. J Biol Chem 2002;277:22789–97. - PubMed
-
- Kambe T, Narita H, Yamaguchi-Iwai Y, et al. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem 2002;277:19049–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
